Abstract
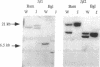
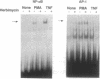
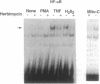
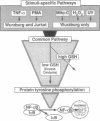
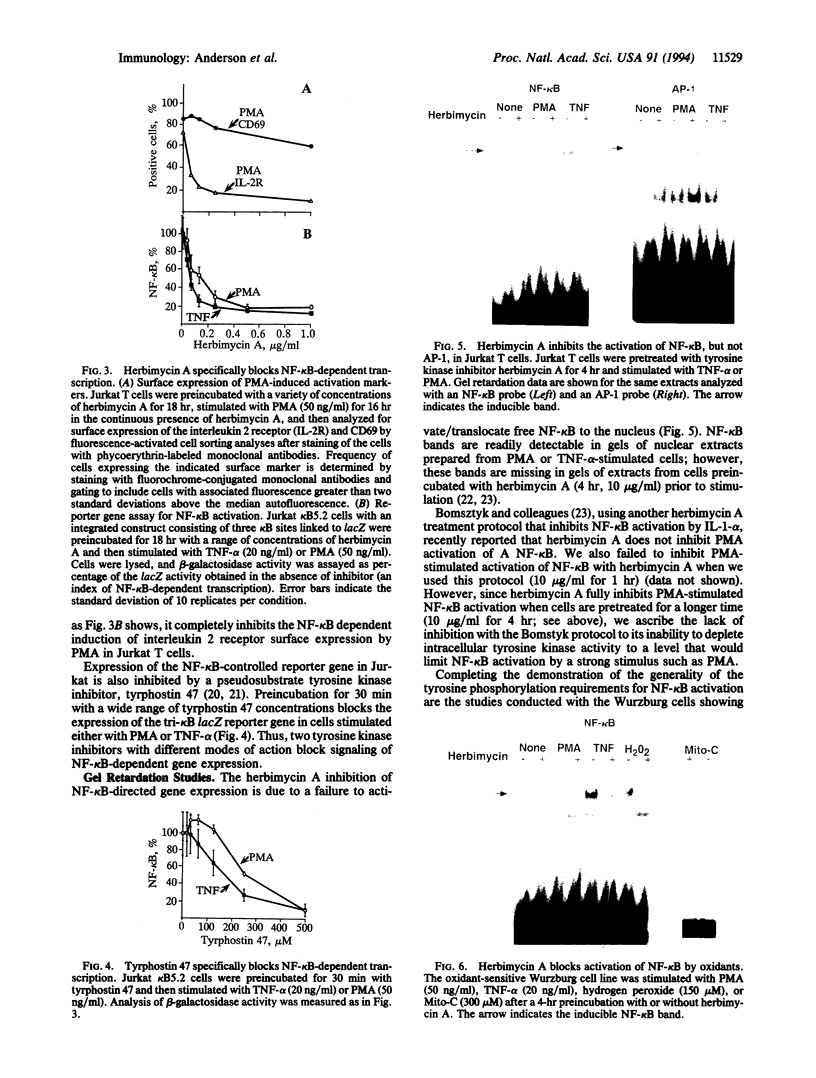
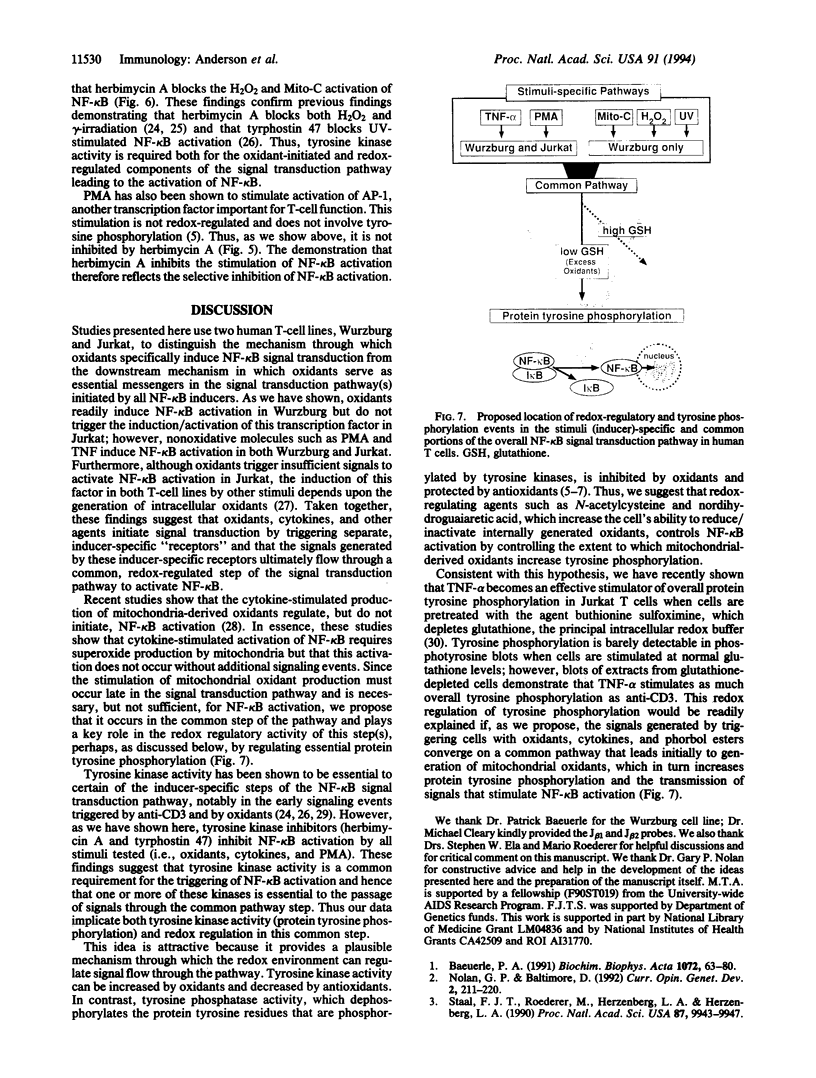
Studies presented here show that overall NF-kappa B signal transduction begins with a parallel series of stimuli-specific pathways through which cytokines (tumor necrosis factor alpha), oxidants (hydrogen peroxide and mitomycin C), and phorbol ester (phorbol 12-myristate 13-acetate) individually initiate signaling. These initial pathways culminate in a common pathway through which all of the stimulating agents ultimately signal NF-kappa B activation. We distinguish the stimuli-specific pathways by showing that the oxidative stimuli trigger NF-kappa B activation in only one of two human T-cell lines (Wurzburg but not Jurkat), whereas tumor necrosis factor alpha and phorbol 12-myristate 13-acetate readily stimulate in both lines. We propose the common pathway as the simplest way of accounting for the common requirements and properties of the signaling pathway. We include a redox-regulatory mechanism(s) in this common pathway to account for the previously demonstrated redox regulation of NF-kappa B activation in Jurkat cells (in which oxidants don't activate NF-kappa B); we put tyrosine phosphorylation in the common pathway by showing that kinase activity (inhibitable by herbimycin A and tyrphostin 47) is required for NF-kappa B activation by all stimuli tested in both cell lines. Since internal sites of oxidant production have been shown to play a key role in the cytokine-stimulated activation of NF-kappa B, and since tyrosine kinase and phosphatase activities are known to be altered by oxidants, these findings suggest that intracellular redox status controls NF-kappa B activation by regulating tyrosine phosphorylation event(s) within the common step of the NF-kappa B signal transduction pathway.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Devary Y., Gottlieb R. A., Smeal T., Karin M. The mammalian ultraviolet response is triggered by activation of Src tyrosine kinases. Cell. 1992 Dec 24;71(7):1081–1091. doi: 10.1016/s0092-8674(05)80058-3. [DOI] [PubMed] [Google Scholar]
- Devary Y., Rosette C., DiDonato J. A., Karin M. NF-kappa B activation by ultraviolet light not dependent on a nuclear signal. Science. 1993 Sep 10;261(5127):1442–1445. doi: 10.1126/science.8367725. [DOI] [PubMed] [Google Scholar]
- Fischer E. H., Charbonneau H., Tonks N. K. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991 Jul 26;253(5018):401–406. doi: 10.1126/science.1650499. [DOI] [PubMed] [Google Scholar]
- Fukazawa H., Mizuno S., Uehara Y. Effects of herbimycin A and various SH-reagents on p60v-src kinase activity in vitro. Biochem Biophys Res Commun. 1990 Nov 30;173(1):276–282. doi: 10.1016/s0006-291x(05)81053-8. [DOI] [PubMed] [Google Scholar]
- Graber M., June C. H., Samelson L. E., Weiss A. The protein tyrosine kinase inhibitor herbimycin A, but not genistein, specifically inhibits signal transduction by the T cell antigen receptor. Int Immunol. 1992 Nov;4(11):1201–1210. doi: 10.1093/intimm/4.11.1201. [DOI] [PubMed] [Google Scholar]
- Israël N., Gougerot-Pocidalo M. A., Aillet F., Virelizier J. L. Redox status of cells influences constitutive or induced NF-kappa B translocation and HIV long terminal repeat activity in human T and monocytic cell lines. J Immunol. 1992 Nov 15;149(10):3386–3393. [PubMed] [Google Scholar]
- Iwasaki T., Uehara Y., Graves L., Rachie N., Bomsztyk K. Herbimycin A blocks IL-1-induced NF-kappa B DNA-binding activity in lymphoid cell lines. FEBS Lett. 1992 Feb 24;298(2-3):240–244. doi: 10.1016/0014-5793(92)80067-q. [DOI] [PubMed] [Google Scholar]
- June C. H., Fletcher M. C., Ledbetter J. A., Schieven G. L., Siegel J. N., Phillips A. F., Samelson L. E. Inhibition of tyrosine phosphorylation prevents T-cell receptor-mediated signal transduction. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7722–7726. doi: 10.1073/pnas.87.19.7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitzki A. Tyrphostins: tyrosine kinase blockers as novel antiproliferative agents and dissectors of signal transduction. FASEB J. 1992 Nov;6(14):3275–3282. doi: 10.1096/fasebj.6.14.1426765. [DOI] [PubMed] [Google Scholar]
- Mattila P. S., Ullman K. S., Fiering S., Emmel E. A., McCutcheon M., Crabtree G. R., Herzenberg L. A. The actions of cyclosporin A and FK506 suggest a novel step in the activation of T lymphocytes. EMBO J. 1990 Dec;9(13):4425–4433. doi: 10.1002/j.1460-2075.1990.tb07893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustelin T., Coggeshall K. M., Isakov N., Altman A. T cell antigen receptor-mediated activation of phospholipase C requires tyrosine phosphorylation. Science. 1990 Mar 30;247(4950):1584–1587. doi: 10.1126/science.2138816. [DOI] [PubMed] [Google Scholar]
- Nolan G. P., Baltimore D. The inhibitory ankyrin and activator Rel proteins. Curr Opin Genet Dev. 1992 Apr;2(2):211–220. doi: 10.1016/s0959-437x(05)80276-x. [DOI] [PubMed] [Google Scholar]
- Northrop J. P., Ullman K. S., Crabtree G. R. Characterization of the nuclear and cytoplasmic components of the lymphoid-specific nuclear factor of activated T cells (NF-AT) complex. J Biol Chem. 1993 Feb 5;268(4):2917–2923. [PubMed] [Google Scholar]
- Schieven G. L., Kirihara J. M., Myers D. E., Ledbetter J. A., Uckun F. M. Reactive oxygen intermediates activate NF-kappa B in a tyrosine kinase-dependent mechanism and in combination with vanadate activate the p56lck and p59fyn tyrosine kinases in human lymphocytes. Blood. 1993 Aug 15;82(4):1212–1220. [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419–6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Osthoff K., Beyaert R., Vandevoorde V., Haegeman G., Fiers W. Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF. EMBO J. 1993 Aug;12(8):3095–3104. doi: 10.1002/j.1460-2075.1993.tb05978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha B. K., Mimnaugh E. G. Free radicals and anticancer drug resistance: oxygen free radicals in the mechanisms of drug cytotoxicity and resistance by certain tumors. Free Radic Biol Med. 1990;8(6):567–581. doi: 10.1016/0891-5849(90)90155-c. [DOI] [PubMed] [Google Scholar]
- Staal F. J., Anderson M. T., Staal G. E., Herzenberg L. A., Gitler C., Herzenberg L. A. Redox regulation of signal transduction: tyrosine phosphorylation and calcium influx. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3619–3622. doi: 10.1073/pnas.91.9.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal F. J., Roederer M., Herzenberg L. A., Herzenberg L. A. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal F. J., Roederer M., Raju P. A., Anderson M. T., Ela S. W., Herzenberg L. A., Herzenberg L. A. Antioxidants inhibit stimulation of HIV transcription. AIDS Res Hum Retroviruses. 1993 Apr;9(4):299–306. doi: 10.1089/aid.1993.9.299. [DOI] [PubMed] [Google Scholar]
- Uckun F. M., Schieven G. L., Tuel-Ahlgren L. M., Dibirdik I., Myers D. E., Ledbetter J. A., Song C. W. Tyrosine phosphorylation is a mandatory proximal step in radiation-induced activation of the protein kinase C signaling pathway in human B-lymphocyte precursors. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):252–256. doi: 10.1073/pnas.90.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara Y., Fukazawa H. Use and selectivity of herbimycin A as inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991;201:370–379. doi: 10.1016/0076-6879(91)01033-x. [DOI] [PubMed] [Google Scholar]
- Uehara Y., Murakami Y., Sugimoto Y., Mizuno S. Mechanism of reversion of Rous sarcoma virus transformation by herbimycin A: reduction of total phosphotyrosine levels due to reduced kinase activity and increased turnover of p60v-src1. Cancer Res. 1989 Feb 15;49(4):780–785. [PubMed] [Google Scholar]
- Vandenberghe P., Verwilghen J., Van Vaeck F., Ceuppens J. L. Ligation of the CD5 or CD28 molecules on resting human T cells induces expression of the early activation antigen CD69 by a calcium- and tyrosine kinase-dependent mechanism. Immunology. 1993 Feb;78(2):210–217. [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Stobo J. D. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J Exp Med. 1984 Nov 1;160(5):1284–1299. doi: 10.1084/jem.160.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]