Abstract
Purpose
Mebendazole (MBZ), first used as an antiparasitic drug, shows preclinical efficacy in models of glioblastoma and medulloblastoma. Three different MBZ polymorphs (A, B and C) exist and a detailed assessment of the brain penetration, pharmacokinetics and anti-tumor properties of each individual MBZ polymorph is necessary to improve mebendazole-based brain cancer therapy.
Experimental Design and Results
In this study, various marketed and custom-formulated MBZ tablets were analyzed for their polymorph content by IR spectroscopy and subsequently tested in orthotopic GL261 mouse glioma model for efficacy and tolerability. The pharmacokinetics and brain concentration of MBZ polymorphs and two main metabolites were analyzed by LC-MS. We found that polymorph B and C both increased survival in a GL261 glioma model, as B exhibited greater toxicity. Polymorph A showed no benefit. Both, polymorph B and C, reached concentrations in the brain that exceeded the IC50 in GL261 cells 29-fold. In addition, polymorph C demonstrated an AUC0-24h brain-to-plasma (B/P) ratio of 0.82, whereas B showed higher plasma AUC and lower B/P ratio. In contrast, polymorph A presented markedly lower levels in the plasma and brain. Furthermore, the combination with elacridar was able to significantly improve the efficacy of polymorph C in GL261 glioma and D425 medulloblastoma models in mice.
Conclusion
Among MBZ polymorphs, C reaches therapeutically effective concentrations in the brain tissue and tumor with less side effects and is the better choice for brain cancer therapy. Its efficacy can be further enhanced by combination with elacridar.
Keywords: Mebendazole, polymorph, glioma, brain distribution, elacridar
Introduction
Central nervous system (CNS) cancers are difficult to treat because most systemically administered therapeutics fail to reach effective concentrations in intracranial tumors (1). This is partially explained by the blood–brain barrier (BBB). In the CNS, the BBB exists along all capillaries consisting of tight junctions thereby blocking large and hydrophilic molecules from passing to the CNS tissues. It is estimated that only about 2% of small-molecule drugs are able to effectively cross the BBB (2).
Mebendazole (MBZ) has been safely used as an antiparasitic in humans for over four decades and displays efficacy against intracranial helminthic infections. We recently demonstrated MBZ preclinical efficacy in orthotopic glioma and medulloblastoma rodent models (3, 4). MBZ significantly reduced tumor growth and improved survival of brain tumor-bearing mice. Based on these results, a Phase I clinical trial with a dose escalation of MBZ for newly diagnosed high-grade glioma patients has been initiated (NCT01729260). Evidence has been generated supporting several anti-cancer mechanisms for MBZ, including tubulin-binding, kinase inhibition, anti-angiogenesis, pro-apoptosis and inhibition of hedgehog pathway (3, 5-9). However, the important features of MBZ's brain penetration and pharmacokinetics remain to be determined. This understanding is important to potentially improve the clinical use of MBZ.
MBZ is highly hydrophobic and can form three different polymorphs based on crystallization conditions (10). The polymorphs A, B and C (MBZ-A, B and C) displayed distinct features in solubility, toxicity and therapeutic effects in anthelmintic applications (11-13). The difference in anti-tumor efficacy of the three polymorphs has not yet been investigated, however, this information might be crucial to future MBZ cancer therapies, since drug formulations might contain various polymorphs in different amounts or combinations. Another critical reason for further investigation is that polymorph C, the most efficacious polymorph in anthelmintic use, can transform over time to the less effective polymorph A, especially with higher temperatures and humidity (14). MBZ polymorphs are three different solid forms that occur depending on which solvent is used during the crystallization of the synthesis process. These structural distinctions in the solid phase disappear once MBZ is dissolved. The variable pharmaceutical properties of MBZ polymorphs are likely the result of different solubility and bioavailability of each polymorph. Since the polymorphs only exist in the solid form and MBZ is exclusively an oral drug, studying the relevant anti-tumor properties of different polymorphs is best accomplished by determining bioavailability and efficacy in animal models via oral administration of MBZ polymorphs.
In this work, we studied pharmacokinetics of MBZ-A, B and C and their concentrations in the brain following the oral administration. We focused our investigation on the brain and brain tumor distributions of MBZ-C, the polymorph found most commonly in the generic MBZ tablets, as well as the levels of MBZ metabolites. To determine if we could further improve MBZ efficacy, we also investigated the use of a P-glycoprotein inhibitor in combination with MBZ.
Materials and Methods
Chemicals and Drugs
MBZ tablets (500 mg) from Janssen Pharmaceuticals (Pantelmin®) and Medley Pharmaceuticals were purchase from local pharmacies in Brazil in 2013 and stored at -20°C freezer. MBZ tablets (100 mg) from Teva Pharmaceuticals USA were purchased from the Outpatient Pharmacy at the Johns Hopkins Hospital in 2011 and stored at room temperature (RT). Teva has discontinued MBZ in the US market since Oct. 2011. Aurochem Laboratories LTD. (Mumbai, India) manufactured MBZ tablets (500mg) S2015 containing the current active pharmaceutical ingredient (API) that typically has mixed polymorphs, and S2017 (polymorph C) with specific API revealed which is the formulation used in the current Phase I clinical trial (NCT01729260). Aurochem also kindly supplied us with MBZ polymorph A, B and C. Elacridar (ELD; GF120918; N-(4-(2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl)phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide)) was purchased from Sigma (St. Louis, MO, USA). Thiabendazole (TBZ), flubendazole (FLZ), oxifendazole (OXZ) and fenbendazole (FBZ) were purchased from Sigma (St. Louis, MO, USA).
Cell Lines and Tissue Culture
Mouse glioma cell line GL261 cells were kindly provided by Dr. Michael Lim's laboratory at the Johns Hopkins University in 2009 and human medulloblastoma cell line D425Med (D425) was obtained from the Duke University Brain Tumor Center without further authentication (3, 15). GL261 and D425 cells were maintained in DMEM media supplemented with 10% fetal bovine serum and antibiotics at 37°C in humidified air containing 5% CO2. GL261-luc cells expressing firefly luciferase were described previously (3).
Cell growth assays
The viable cells were measured with Cell Counting Kit-8 (Dojindo Molecular Technology, Japan) containing WST-8 at 450 nm on a PerkinElmer VICTOR3 plate reader. IC50s measurements were perfomed by incubating cells at a range of concentrations for 72 h and calculated by GraphPad Prism 5.0 using the log(inhibitor) vs. response function and non-linear fit.
Infrared Spectrometry of MBZ polymorphs
A Direct Detect™ infrared (IR) spectrometer was used (Millipore, Billerica, MA, USA). MBZ powder or tablets ground to powder was mixed with water first, applied to the card and air dried following the manufacturer's instructions. The spectra of –C=O and –NH were analyzed and compared as described before (10).
Intracranial Mouse Models
All animal studies were approved by the Animal Care and Use Committee (ACUC) of the Johns Hopkins University. The intracranial implantation of GL261-luc in the frontal lobe and D425 cells in the cerebellum of the mouse brain followed the procedure described before (3, 4). Five days after tumor implantation, mice were gavaged with MBZ or the other benzimidazoles at 50 mg/kg daily for the first 20 days and then five days a week as described before for single drug treatment (3). MBZ and other benzimidazoles were prepared by either mixing the power with PBS and sesame oil (1:1, v:v) (Sigma) or by grinding the tablets to powder and resuspending in the aforementioned PBS/ sesame oil mixture. Elacridar was prepared as a 10 mg/ml suspension in 0.5% hydroxypropylmethylcellulose and 0.5% Tween 80 in PBS similarly as described before (16).
MBZ Pharmacokinetic Studies
Female C57BL6 mice, 5-6 weeks of age, were purchased from NCI. Animal experimentation was conducted under an approved IACUC protocol and complied with local and national guidelines. All MBZ polymorphs were administered by oral gavage at a dose of 50 mg/kg. Elacridar was administered by oral gavage at 50 mg/kg 2 hours prior to the administration of MBZ-C. Mice (3 animals/time point) were first anesthetized via intraperitoneal injection of 60 μl of a stock solution containing ketamine hydrochloride (75 mg/kg) (100 mg/ml; Ketamine HCl; Abbot Laboratories, Chicago, IL, USA) and xylazine (7.5 mg/kg) (100 mg/ml; Xyla-ject®; Phoenix Pharmaceutical, St. Joseph, MO, USA) in a sterile 0.9% NaCl solution. Then the blood samples were taken by puncturing and aspiring from the left heart ventricle. Blood samples were mixed with 5 mM EDTA and centrifuged at 10000 g for 5 min to obtain the plasma for further analysis.
For brain distribution studies, mice were perfused under anesthesia with 20 ml ice-cold saline supplemented with 20 μl of 0.02% heparin by injecting slowly into the left heart ventricle using a 20 gauge needle. The right atrium was cut open before to allow the blood outflow. The yellow color of kidney indicated a good perfusion quality that was essential to deplete blood from the brain tissue. In GL261 tumor-bearing mice, GL261 tumor was distinguished from the normal brain by easily recognizable differences in color and shade. GL261 tumor was separated with a scalpel and the normal brain tissue was cut from the contralateral hemisphere. All brain samples were weighed and stored at -80°C before processing.
Blood, brain and brain tumor tissues were harvested as a function of time after MBZ administration. To compare the pharmacokinetics of MBZ polymorphs, three cohorts of mice each were administered a single dose of 50 mg/kg by oral gavage. For the initial comparison studies, plasma samples were obtained at 1, 2.5, 4, 6, 8, 15, and 24 hours after MBZ administration while brain tissue was only collected at 6 hours. For the comparison studies of polymorph C with or without ELD, plasma and brain tissue samples were obtained at 2.5, 4, and 8 hours after MBZ administration. Brain tumor tissue samples were also obtained for polymorph C alone.
Measurement of MBZ and Metabolites
MBZ and the two metabolites, 2-amino-5-benzoyl-benzimidazole (MBZ-NH2, CAS 52329-60-9) and rac dihydro mebendazole (MBZ-OH, CAS 60254-95-7), were quantified in plasma, brain and brain tumor tissue. Tissue homogenates were prepared at a concentration of 200 mg/ml in plasma prior to extraction. Mebendazole and metabolites were extracted from 50 μl of plasma or tissue homogenates with 0.1 ml of methanol containing 0.5 μg/ml of the internal standard A620223.69. After centrifugation, the supernatant (60 μl) was mixed with water (40 μl) and then transferred into autosampler vials. Separation was achieved with an Atlantis dC18 (2.1 x 100mm, 3 μm) column at room temperature with methanol/water mobile phase (60:40, v:v) containing 0.1% formic acid using isocratic flow at 0.25 ml/min for 5 minutes. The analytes were monitored using an AB Sciex triple quadrapoleTM 5500 mass-spectrometric detector (Applied Biosystems, Foster City, CA, USA) using electrospray ionization operating in positive mode. The spectrometer was programmed to allow the [MH+] ions of MBZ, MBZ-NH2, MBZ-OH, and A620223.69 at m/z 296.0, 238.0, 298.0, and 287.2, respectively to pass through the first quadrupole (Q1) and into the collision cell (Q2). The daughter ions for MBZ (m/z 263.9), MBZ-NH2 (m/z 105.1), MBZ-OH (m/z 266.0), and A620223.69 (m/z 124.1) were monitored through the third quadrupole (Q3). Calibration curves for MBZ and metabolites were computed using the area ratio peak of the analysis to the internal standard by using a quadratic equation with a 1/x weighting function over the range of 5 to 500 ng/ml (MBZ) and 1 to 500 ng/ml (metabolites) with dilutions of up to 1:100 (v:v). If one or more concentrations were below limits of quantification, a value of ½ the limit of quantification was assigned for pharmacokinetic calculations. If two consecutive time points were below limits of quantification, the last one was excluded from the analysis.
Mean plasma and brain concentrations were calculated at each time point for both MBZ and its metabolites. 1.045 g/ml was used as the average wet rodent brain tissue density (17). Pharmacokinetic parameters were calculated from mean MBZ and its metabolites concentration-time data using noncompartmental methods as analyzed in Phoenix ® WinNonlin® version 6.3 (Pharsight Corp., Mountain View, CA). Cmax and Tmax were the observed values from the mean concentration data. The AUClast was calculated using the log-linear trapezoidal method. λz was determined from the slope of the terminal phase of the concentration-time profile. The terminal half-life (T1/2) was determined by dividing 0.693 by λz. If the r2 of λz was <0.9, the T1/2 was not reported. Relative systemic exposure to MBZ was calculated using the AUClast: Metabolites AUClast/MBZ AUClast. Relative systemic exposure in brain or brain tumor compared with plasma was calculated using the AUClast: Brain or Brain Tumor AUClast/ Plasma AUClast.
Statistical Analysis
Animal survival data were analyzed by GraphPad Prism 5.0. The p-values were determined by a Mantel-Cox test. A p-value under 0.05 was accepted as statistically significant.
For the pharmacokinetic studies comparing the polymorphs or administration with ELD, the Method of Bailer was used to estimate the variance of AUClast given the calculated variance of the mean concentration at each time point (18). This was then followed by a pairwise comparison using a Z-test to determine whether there was a significant difference between MBZ exposure as expressed by AUClast (19). Comparisons of individual data were conducted using the nonparametric Wilcoxon signed rank test with post-hoc analysis using an All Pairs Tukey-Kramer test. The level of significance was P<0.05.
Results
Polymorph C was most effective for treating brain tumors in mice
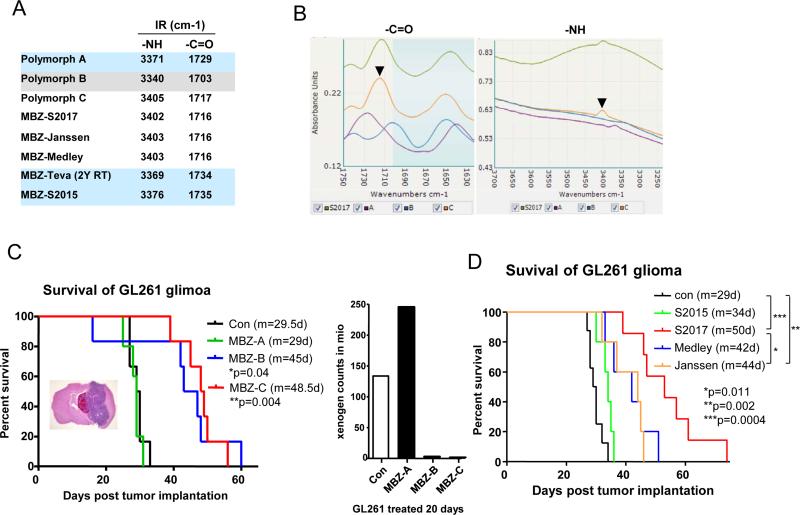
We examined the polymorph content of several commercially available tablets (Janssen, Medley and Teva) and two made to order tablets (Aurochem S2015 used the current API that typically has mixed polymorphs and S2017 was specified as pure MBZ-C) by comparing their IR profiles with the individual MBZ polymorphs (Figure 1A and B). Based on the IR peaks of –C=O and –NH bonds, we determined that the Janssen and Medley tablets were made of mainly MBZ-C as well as the Aurochem S2017. Aurochem S2015 and Teva tablets that have been stored at RT for 2 years showed mainly the profiles of MBZ-A. As a control, polymorph A, B and C were dissolved in DMSO and incubated individually with GL261 glioma cells, which showed equal cytotoxicity (data not shown).
Figure 1. MBZ-C is the most efficacious polymorph with limited toxicity.
A and B. Infra-red spectra of MBZ polymorphs and MBZ tablets from different suppliers: S2015 and S2017 (Aurochem), Teva (after 2-year storage at RT), Medley and Janssen. Two peaks represent the -NH and –C=O groups in the molecules. Black arrow heads indicate the peaks of MBZ-C control. C. (Left panal) Kaplan Meier survival curves of mice implanted with GL261-luc glioma and treated with different MBZ polymorphs (A, B and C). A H&E staining of the GL261-luc glioma-bearing mouse brain by coronal cut was shown. 5 days after the tumor implantation, the mice were gavaged with MBZ and control animals were feed with vehicles. One mouse in the MBZ-B group presumably died from drug toxicity as no significant tumor was found in the brain. The p-values of Con vs MBZ-B and Con vs MBZ-C are indicated. The p-value of MBZ-B vs MBZ-C is 0.72. m: median survival in days. Con: n=6; MBZ-A: n=5; MBZ-B: n=6; MBZ-C: n=6. (Right panel) Luciferase counts measured by Xenogen reflected the size of GL261-luc brain tumor in mice treated with MBZ polymorphs for 20 days. D. Survival curves of GL261-luc bearing mice treated with MBZ tablets from different suppliers. Con: n=6; S2015: n=5; S2017: n=6; Medley: n=5; Janssen: n=5.
MBZ-A appeared to be ineffective in treating intracranial GL261 glioma-bearing mice, while MBZ-C displayed the best efficacy (Figure 1C). Although MBZ-B showed a similar survival to MBZ-C, it caused more toxicity with 1 treatment-related death among 6 treated mice (Figure 1C, left panel) and loss of body weight (Supplementary Figure 1). The efficacy data reflected the polymorph composition of MBZ tablets well in the sense that S2015 was ineffective and other tablets made of MBZ-C all showed significant efficacy by extending the mean survival to 42-50 days from 29 days of the control group (Figure 1D).
MBZ reached the brain at significant levels
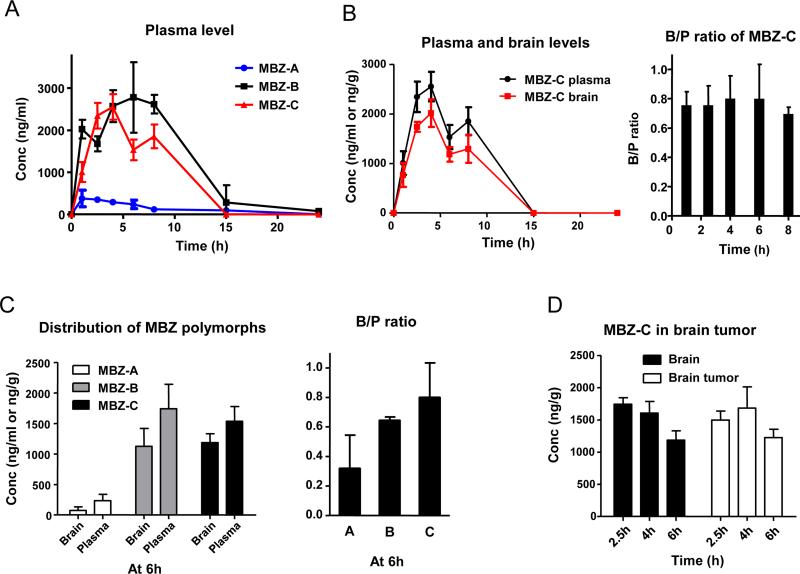
Following an oral dosing of 50 mg/kg, MBZ-C achieved a plasma AUC0-24h of 16,039 h*ng/ml (Figure 2A and Table 1). In comparison, MBZ-B reached a plasma AUC0-24h of 26,474 h*ng/ml, while MBZ-A plasma AUC0-24h reached only 3,052 h*ng/ml, by far the lowest among all three polymorphs (P<0.05 for AUC0-24h with MBZ-B>-C>-A; Table 1). Measurements of brain tissues following a thorough perfusion revealed significant presence of MBZ-C over a time course, correlating closely with the plasma MBZ levels with a brain/plasma (B/P) ratio of 0.75 on average that remained relatively stable during the 8 hours (Figure 2B). Comparing the polymorphs at 6 h following oral gavage, we found MBZ-C and –B achieved similar brain levels, despite MBZ-B's higher levels and AUC0-24h in the plasma (Figure 2C left panel and Table 1), resulting in a slightly favorable mean B/P ratio of MBZ-C over MBZ-B (0.80 for C vs 0.64 for B and 0.29 for A, p=0.055) (Figure 2C right panel). This corroborates well with the efficacy data in Figure 1C, where MBZ-B and –C demonstrated similar survival benefit in GL261 model (mean survival: 45 days of MBZ-B vs 48.5 days of MBZ-C). However, it is notable that MBZ-B displayed greater toxicity, resulting in early death of one mouse among the six treated animals (Figure 2C left panel). Analysis of the GL261 brain tumor and the contralateral brain tissues indicated equal distribution of MBZ-C in the brain tumor and the normal brain tissues (Figure 2D).
Figure 2. Plasma and brain distributions of MBZ polymorphs.
A. A time course of the MBZ plasma levels in C57BL6 mice after oral gavage of MBZ-A, B or C at 50mg/kg.
B. (Left panel) Brain and plasma levels of MBZ-C in a time course after oral gavage at 50mg/kg. Animals were thoroughly perfused with PBS for all brain distribution studies. (Right panel) Brain/plasma (B/P) ratios of MBZ-C. Data were collected from three mice at each time point.
C. (Left panel) Brain and plasma levels of MBZ polymorphs at 6 h following oral gavage (50mg/kg). B/P ratio of MBZ polymorphs at 6 h following oral gavage. (Right panel) The mean B/P ratio of MBZ-A is 0.32, MBZ-B is 0.64 and MBZ-C is 0.80.
D. MBZ-C distributed equally in the brain and brain tumor. GL261 tumors implanted in the right side of mouse frontal lobe were resected and compared with the contralateral normal brain tissue.
Table 1.
Pharmacokinetics of MBZ polymorphs in mice.
| Plasma | Brain | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Polymorph | Detection | T1/2 (h) | Tmax (h) | Cmax (ng/ml) | AUC0-24h (h*ng/ml) | T1/2 (h) | Tmax (h) | Cmax (ng/g) | AUC0-24h (h*ng/g) | B/P |
| A | MBZ | 3.23 | 1 | 379.3 | 3052 | |||||
| B | MBZ | 3.18 | 6 | 2778.3 | 26474 | |||||
| C | MBZ | 0.90 | 4 | 2553.3 | 16039 | 1.64 | 4 | 2016 | 13134 | 0.82 |
| A | MBZ-NH2 | 2.5 | 201.3 | 2841 | ||||||
| B | MBZ-NH2 | 8 | 1656.7 | 18583 | ||||||
| C | MBZ-NH2 | 8 | 1416.7 | 10516 | 8 | 201.6 | 1336 | 0.13 | ||
| A | MBZ-OH | 1 | 32.1 | 247 | ||||||
| B | MBZ-OH | 8 | 744.7 | 4970 | ||||||
| C | MBZ-OH | 6 | 951.7 | 5781 | 8 | 794.3 | 5427 | 0.94 | ||
| Plasma | Brain | B/P | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Drug | Detection | Tmax (h) | Cmax (ng/mL) | AUC0-8h (h*ng/mL) | Tmax (h) | Cmax (ng/g) | AUC0-8h (h*ng/g) | AUC0-8h | Significant? |
| MBZ | MBZ | 4 | 2553.3 | 15340 | 4 | 2016.0 | 11510 | 0.75 | |
| ELD+MBZ | MBZ | 2.5 | 1433.3 | 8636 | 2.5 | 1459.7 | 8904 | 1.03 | No |
| MBZ | MBZ-NH2 | 8 | 1416.7 | 8264 | 8 | 201.6 | 1010 | 0.12 | |
| ELD+MBZ | MBZ-NH2 | 2.5 | 1191.7 | 7826 | 8 | 387.8 | 2386 | 0.30 | Yes |
| MBZ | MBZ-OH | 2.5 | 810.0 | 4679 | 8 | 794.3 | 4135 | 0.88 | |
| ELD+MBZ | MBZ-OH | 2.5 | 491.0 | 2680 | 2.5 | 712.6 | 3475 | 1.30 | No |
Pharmacokinetics of MBZ polymorphs in mice.
Pharmacokinetics of MBZ metabolites
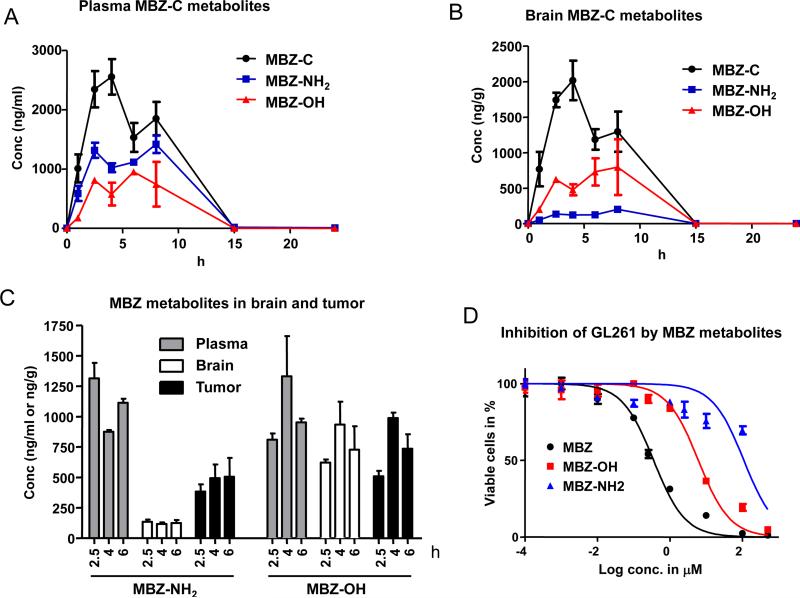
We determined the plasma levels of the major metabolites MBZ-NH2 and MBZ-OH of MBZ polymorphs (P<0.05 for AUC0-24h of MBZ-NH2 with MBZ-B>C>A; P<0.05 for AUC0-24h of MBZ-OH with MBZ-B and C>A; Table 1). The levels of MBZ-C's metabolites in plasma and brain generally followed the same pattern of MBZ-C's concentration (Figure 3A and B). MBZ-NH2 showed higher levels than MBZ-OH in the plasma (Figure 3A), with an AUC0-24h of 10,516 h*ng/ml compared to 5,781 h*ng/ml of MBZ-OH (Table 1). Notably, in a reversed pattern, MBZ-NH2 was measured at much lower levels than MBZ-OH in the brain in terms of Cmax and AUC0-24h (Figure 3B and Table 1). Interestingly, MBZ-NH2 reached significantly higher levels in GL261 glioma than in the contralateral brain (Figure 3C). In order to elucidate the anti-tumor role of MBZ metabolites, we compared the IC50 of MBZ, MBZ-OH and MBZ-NH2 in GL261 cells and determined MBZNH2 is the least cytotoxic derivative of MBZ in vitro (Figure 3D).
Figure 3. Distribution of MBZ Metabolites.
A and B. The plasma and brain levels of MBZ and its metabolites, MBZ-OH (rac dihydro mebendazole, CAS 60254-95-7) and MBZ-NH2 (2-amino-5-benzoylbenzimidazole, CAS 52329-60-9), were analyzed following oral gavage of 50mg/kg MBZ-C.
C. The distributions of MBZ metabolites in the plasma, brain and GL261 brain tumor. At 2.5, 4 and 6 hours after oral gavage of MBZ-C, mice implanted with GL261 for 25 days were sacrificed and the blood, GL261 brain tumor and contra-lateral normal brain tissues were sampled and analyzed.
D. IC50 curve of GL261 giloma cells with MBZ and metabolites. GL261 cells were incubated with MBZ or its metabolites for 72 h and the living cells were measured.
Combination of MBZ with elacridar
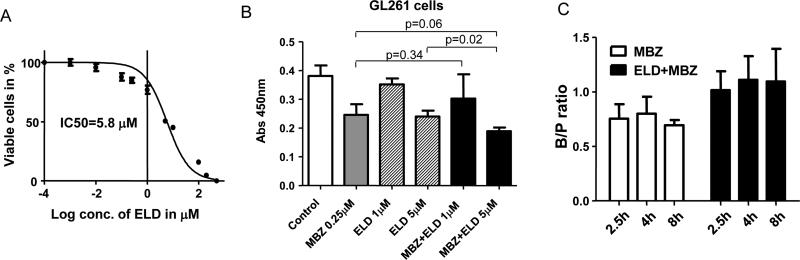
Achieving a sufficient therapeutic concentration in the tumor and the surrounding brain tissue is a critical challenge that is faced by almost all brain cancer therapies. Four hours after oral administration, we found MBZ-C brain concentration peaked at 2,016 ng/g (equivalent to 7.1 μM) (Table 1), which was well above the IC50s of cultured glioma and medulloblastoma cells (0.11-1 μM) and also above MBZ's inhibitory IC50 with VEGFR2 kinase at 4.3 μM in vitro (3, 4). The relatively high brain concentration might help explain MBZ efficacy in brain tumor models. Next, we reasoned that a further increase in the brain distribution of MBZ would be desirable as it may increase therapeutic efficacy. Aside from a pure mechanical barrier, the BBB employs active efflux mechanisms to limit drug entry such as P-glycoprotein (P-gp). Elacridar (ELD) is a potent third-generation inhibitor that inhibits P-gp as well as breast cancer resistance protein (BCRP) and co-administration of elacridar has increased the brain penetration of several drugs (16, 20). We first examined the cytotoxicity of elacridar in GL261 mouse glioma cells and determined the IC50 to be 5.8 μM (Figure 4A). Combining elacridar with 0.25 μM MBZ only marginally increased the cytotoxicity in vitro (Figure 4B). Oral administration of 50 mg/kg elacridar two hours prior to MBZ-C did not significantly change the brain concentration of MBZ in terms of AUC0-8h, while B/P ratio average of 2.5, 4 and 8h was shifted slightly higher from 0.75 to 1.03, which, however, was not statistically significant (Table 1 and Figure 4C). Interestingly, this was accompanied by a significant increase in MBZ-NH2 along with an elevation of the B/P ratio from 0.12 to 0.30 in the brain when treated with a combination of elacridar and MBZ-C (Table 1).
Figure 4. Combination of MBZ with elacridar.
A. IC50 curve of GL261 glioma cells with elacridar (ELD). IC50=5.8 μM.
B. Inhibition of GL261 cells by MBZ (0.25 μM), ELD (1 or 5 μM) or the combination. Cells were incubated with the indicated drugs for 72 h and the living cells were measured by the colorimetric assay.
C. ELD elevated the average B/P ratios of MBZ in mice.
Combination with elacridar improved the treatment of MBZ
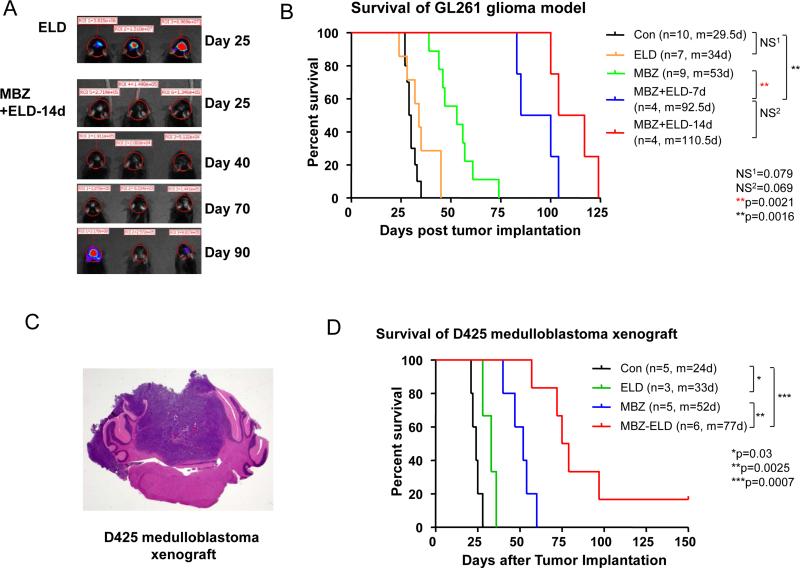
Combination therapy of elacridar and MBZ increased the survival benefit in GL261 syngeneic glioma and D425 xenograft medulloblastoma models (Figure 5). This was achieved by adding 7 or 14 days of 50 mg/kg elacridar treatment to the standard MBZ (MBZ-C) regimen of 50 mg/kg. Specifically, in GL261, combination therapy improved the median survival to 92.5 and 110.5 days dependent on the treatment length, which is a stark increase from 53 days of MBZ alone as well as 29.5 days (control) and 34 days (elacridar alone) (Figure 5B). Similarly, in the orthotopic D425 medulloblastoma xenograft model, the combination of elacridar with MBZ increased the median survival to 77 days (Figure 5D). This is a significant improvement from MBZ only treatment with 52 days of survival and elacridar alone, which showed a marginal survival benefit of 9 days in this particular animal model.
Figure 5. Combination of MBZ with elacridar improved the efficacy.
A and B. GL261 cells transfected with luciferase were implanted in C57BL6 mice and the treatments were initiated 5 days after the implantation. Elaridar (ELD) was oral gavaged at 50 mg/kg 2 hours before the MBZ administration (50 mg/kg) for the first 7 or 14 days of treatment. Thereafter, MBZ was given five days a week at the same dose for the rest of the therapy. The ELD alone group was gavaged with ELD for 14 daily doses. Animals treated by MBZ and ELD were monitored by Xenogen for tumor luciferase signals starting from 25 days after the tumor implantation (A).
C and D. D425 medulloblastoma cells were implanted in the mouse cerebellum and formed a cerebellar tumor (C, H&E staining). Five days after the tumor implantation, mice were treated with vehicle (Con), 7 days of 50 mg/kg elacridar (ELD), 50 mg/kg MBZ-C alone (MBZ) or 7 days of 50mg/kg ELD with 50 mg/kg MBZ-C (MBZ-ELD) following the same dosing regime in B.
A prolonged treatment course with elacridar and MBZ was attempted, however, increased toxicity such as severe weight loss and mortality limited those studies (data not shown).
Discussion
The limited ability of cancer therapeutics to accumulate in the tumor is a major obstacle for improving brain cancer therapy (1). While the pharmacokinetics of MBZ for antiparasitic use have been studied in human and a number of animals, there is no CNS distribution data, especially in relation to efficacy against brain cancer (21). This is important for advancing MBZ as a potential anti-brain cancer drug (3).
In this study, we demonstrate that MBZ can reach the brain tissue in significant concentrations and with high brain to plasma ratios. Between 1 and 8 hours after the oral administration, MBZ-C maintained the brain levels above 0.767 μg/g (equivalent to 2.7 μM), with a Cmax of 2,016 μg/g (equivalent to 7.1 μM). This exceeded the IC50 (4.3 μM) of MBZ on VEGFR2 kinase in vitro and the IC50 (0.11-1 μM) in a series of glioma and medulloblastoma cell lines in tissue culture (3, 4). Furthermore, MBZ-C emerged as the most efficient polymorph, achieving an AUC0-24h B/P ratio of 0.82. This is encouraging since temozolomide, the standard treatment for high-grade gliomas, was measured of having a B/P ratio of 0.408 in mice and a cerebrospinal fluid (CSF)/plasma ratio of 0.2 in human (22, 23). In our study, the distributions of MBZ in the GL261 brain tumor and in the normal brain tissue did not differ significantly. It is worth mentioning that advanced growth of GL261 glioma results in substantial amount of blood in the tumor, similarly to other glioma models and a thorough perfusion was essential to eliminate the contamination of MBZ from the blood. It is noteworthy that in some human trials MBZ achieved lower plasma levels than in mice as the amino and hydroxy metabolites reached two- to ten-fold the plasma levels of the parental MBZ (24, 25), which could indicate higher metabolic enzyme activities in human. Thus, much higher doses up to 200 mg/kg/day could be tolerated in human and should be used in future human brain tumor therapy (26).
Among the three polymorphs, MBZ-A showed no efficacy in GL261 glioma model, explained by the very low plasma presence at only 19% of AUC0-24h measured with MBZ-C. MBZ-A's low bioavailability and inferior anti-tumor efficacy are in line with previous reports of its poor performance in anti-parasitic applications (11, 13). In comparison, MBZ-B was able to reach 165% of MBZ-C's AUC0-24h in the plasma, while showing a similar brain concentration demonstrated by the measurement at 6 h. This could explain the elevated toxicity of MBZ-B in GL261 glioma-bearing mice as the anti-brain tumor efficacy remained essentially the same compared to MBZ-C. Thus, we suggest that MBZ-C is a better choice in brain tumor therapy. As a practical matter, the tablets made of MBZ-C should be stored under lower temperature (14), since the MBZ tablets of Teva brand may have lost its efficacy under the standard RT condition within 3 years likely due to the conversion to polymorph A, although we do not know the original concentration of polymorph C in these tablets that used to be efficacious in our previous study (3).
MBZ's small size (295 daltons) and lipophilic property favor brain penetration (2). It is remarkable that other benzimidazoles tested so far, such as albendazole, thiabendazole, flubendazole, oxifendazole and fenbendazole sharing similar physical properties, failed or only marginally improved the survival of GL261 glioma-bearing mice, even at higher doses than MBZ (Supplementary Figure 2) (3). As we previously made the observation that fenbendazole in feed impaired the intake of the implantation of a medulloblastoma cell line in athymic nude mice (3), it only made a very marginal and statistically insignificant survival improvement in GL261 glioma model by gavaging 5 days after the implantation. There are several factors potentially contributing to the stark discrepancy in the brain tumor therapy with various benzimidazoles. For one as shown with MBZ polymorph A, low bioavailability likely due to the poor absorption could be detrimental to the therapeutic performance of this class of drugs. Second, the brain penetration of these benzimidazoles has not been well studied and could be insufficient for any significant therapeutic effects. Furthermore, MBZ has been implicated in inhibiting multiple tyrosine kinases in recent reports, whereas albendazole showed lack of such ability, indicating differences in anti-tumor mechanisms among benzimidazoles (4, 7, 8).
P-glycoprotein (P-gp, ABCB1) is an ATP-binding cassette (ABC) transporter and plays an important role in limiting drug uptake into the brain.(27) Elacridar is a 3rd generation inhibitor of P-gp efflux transporters and also inhibits the breast cancer-resistant protein (BCRP, ABCG2) that is another key efflux transporter in BBB, as well as organic anion-transporting polypeptide 1B1 (OATP1B1), a hepatic uptake transporter (28, 29). Furthermore, elacridar has been found safe in Phase I clinical trials (20). In this study, we investigated the combination of elacridar with MBZ to potentially enhance its therapeutic efficacy. As results, we found that the combination greatly improved the survival in two orthotopic brain tumor models. However, in this limited study, the B/P ratio and brain AUC0-8h of MBZ did not show statistically significant differences with co-administration of elacridar, despite its ability to significantly increase survival in brain cancer bearing mice. When analyzing the metabolites, MBZ-NH2, one of the two major metabolites in rodents and human (21), was significantly elevated in terms of B/P ratio (2.5 folds) and AUC0-8h (2.4 folds) as a result of co-administration of elacridar. Also noticeable is our finding that MBZ-NH2 was preferentially accumulated in the GL261 brain tumor vs the normal brain tissues. Although these data could indicate that MBZ-NH2 is a potential substrate of P-gp and/or ABCG2, the significance of this finding is unclear at this point. A possible direct cytotoxic effect of MBZ-NH2 appears unlikely as further testing displayed only a marginal cytotoxicity with cultured GL261 cells. The treatment of MBZ-NH2 in GL261 brain tumor-bearing mice failed to show conclusive results (Supplementary Figure 3). On the other hand, the added cytotoxic effects of MBZ and elacridar could also contribute to the enhanced efficacy. In D425 medulloblastoma cells, elacridar at 5 μM significantly enhanced the cytotoxicity of MBZ in vitro (Supplementary Figure 4). Further investigations include the study of MBZ and elacridar interactions, particularly the potential substrate profile of efflux transporters with MBZ, in order to better understand and thereby improve the combination with MBZ.
In conclusion, MBZ-C is the most efficacious polymorph in brain tumor therapy. The combination of MBZ-C with elacridar can greatly improve the efficacy and this combination should be studied for safety in future Phase I clinical trials of high grade glioma and/or medulloblastoma.
Supplementary Material
Translational Relevance.
Mebendazole is an antiparasitic drug with over 40 years of safe use. We recently repurposed mebendazole for glioblastoma therapy and launched a Phase I trial. In preclinical studies, mebendazole increases survival in multiple orthotopic glioma and medulloblastoma models. Three polymorphs of mebendazole exist, but the relative polymorph content for existing drugs varies, and the therapeutic relevance of the different polymorphs to anti-cancer activity is unknown. We show that as an oral drug mebendazole polymorph C is superior form and it reaches the brain and brain tumors in effective concentrations. Efficacy was further improved by combining mebendazole with elacridar. We recommend using pure polymorph C for future brain cancer clinical trials, possibly in combination with elacridar.
Acknowledgement
Funding of this study was from the Accelerate Brain Cancer Cure foundation to GJR, the Virginia and D.K. Ludwig Fund for Cancer Research, generous support from Peter Jennison, the Irving J Sherman Research Professorship in Neurosurgery to GJR and National Institute of Neurological Disorders and Stroke (R25NS065729) to VS, NIH P30-CA006973, UL1-RR025005 and 1S10RR026824-01 to MAR. BRY, VS, AJ and GJR are the inventors of a provisional patent application titled “Mebendazole Polymorph for Brain Tumors”.
Footnotes
Disclosure of Potential Conflicts of Interest:
BRY, VS, AJ and GJR are the inventors of a provisional patent application titled “Mebendazole Polymorph for Brain Tumors”.
References
- 1.Bai RY, Staedtke V, Riggins GJ. Molecular targeting of glioblastoma: Drug discovery and therapies. Trends Mol Med. 2011;17:301–12. doi: 10.1016/j.molmed.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chico LK, Van Eldik LJ, Watterson DM. Targeting protein kinases in central nervous system disorders. Nat Rev Drug Discov. 2009;8:892–909. doi: 10.1038/nrd2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai RY, Staedtke V, Aprhys CM, Gallia GL, Riggins GJ. Antiparasitic mebendazole shows survival benefit in 2 preclinical models of glioblastoma multiforme. Neuro Oncol. 2011;13:974–82. doi: 10.1093/neuonc/nor077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai RY, Staedtke V, Rudin CM, Bunz F, Riggins GJ. Effective treatment of diverse medulloblastoma models with mebendazole and its impact on tumor angiogenesis. Neuro Oncol. 2014 doi: 10.1093/neuonc/nou234. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doudican N, Rodriguez A, Osman I, Orlow SJ. Mebendazole induces apoptosis via Bcl-2 inactivation in chemoresistant melanoma cells. Mol Cancer Res. 2008;6:1308–15. doi: 10.1158/1541-7786.MCR-07-2159. [DOI] [PubMed] [Google Scholar]
- 6.Doudican NA, Byron SA, Pollock PM, Orlow SJ. XIAP downregulation accompanies mebendazole growth inhibition in melanoma xenografts. Anticancer Drugs. 2013;24:181–8. doi: 10.1097/CAD.0b013e32835a43f1. [DOI] [PubMed] [Google Scholar]
- 7.Dakshanamurthy S, Issa NT, Assefnia S, et al. Predicting new indications for approved drugs using a proteochemometric method. J Med Chem. 2012;55:6832–48. doi: 10.1021/jm300576q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nygren P, Fryknas M, Agerup B, Larsson R. Repositioning of the anthelmintic drug mebendazole for the treatment for colon cancer. J Cancer Res Clin Oncol. 2013;139:2133–40. doi: 10.1007/s00432-013-1539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsen AR, Bai RY, Chung JH, et al. Repurposing the antihelmintic mebendazole as a hedgehog inhibitor. Mol Cancer Ther. 2015;14:3–13. doi: 10.1158/1535-7163.MCT-14-0755-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liebenberg W, Dekker TG, Lotter AP, de Villiers MM. Identification of the mebendazole polymorphic form present in raw materials and tablets available in South Africa. Drug Dev Ind Pharm. 1998;24:485–8. doi: 10.3109/03639049809085647. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Caabeiro F, Criado-Fornelio A, Jimenez-Gonzalez A, et al. Experimental chemotherapy and toxicity in mice of three mebendazole polymorphic forms. Chemotherapy. 1987;33:266–71. doi: 10.1159/000238506. [DOI] [PubMed] [Google Scholar]
- 12.Swanepoel E, Liebenberg W, de Villiers MM. Quality evaluation of generic drugs by dissolution test: changing the USP dissolution medium to distinguish between active and non-active mebendazole polymorphs. Eur J Pharm Biopharm. 2003;55:345–9. doi: 10.1016/s0939-6411(03)00004-3. [DOI] [PubMed] [Google Scholar]
- 13.Charoenlarp P, Waikagul J, Muennoo C, Srinophakun S, Kitayaporn D. Efficacy of single-dose mebendazole, polymorphic forms A and C, in the treatment of hookworm and Trichuris infections. Southeast Asian J Trop Med Public Health. 1993;24:712–6. [PubMed] [Google Scholar]
- 14.Brits M, Liebenberg W, de Villiers MM. Characterization of polymorph transformations that decrease the stability of tablets containing the WHO essential drug mebendazole. J Pharm Sci. 2010;99:1138–51. doi: 10.1002/jps.21899. [DOI] [PubMed] [Google Scholar]
- 15.Bai RY, Staedtke V, Lidov HG, Eberhart CG, Riggins GJ. OTX2 Represses Myogenic and Neuronal Differentiation in Medulloblastoma Cells. Cancer Res. 2012;72:5988–6001. doi: 10.1158/0008-5472.CAN-12-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sane R, Agarwal S, Elmquist WF. Brain distribution and bioavailability of elacridar after different routes of administration in the mouse. Drug Metab Dispos. 2012;40:1612–9. doi: 10.1124/dmd.112.045930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiResta GR, Lee J, Lau N, Ali F, Galicich JH, Arbit E. Measurement of brain tissue density using pycnometry. Acta Neurochir Suppl (Wien) 1990;51:34–6. doi: 10.1007/978-3-7091-9115-6_12. [DOI] [PubMed] [Google Scholar]
- 18.Bailer AJ. Testing for the equality of area under the curves when using destructive measurement techniques. J Pharmacokinet Biopharm. 1988;16:303–9. doi: 10.1007/BF01062139. [DOI] [PubMed] [Google Scholar]
- 19.Yuan J. Estimation of variance for AUC in animal studies. J Pharm Sci. 1993;82:761–3. doi: 10.1002/jps.2600820718. [DOI] [PubMed] [Google Scholar]
- 20.Werle M, Takeuchi H, Bernkop-Schnurch A. New-generation efflux pump inhibitors. Expert Rev Clin Pharmacol. 2008;1:429–40. doi: 10.1586/17512433.1.3.429. [DOI] [PubMed] [Google Scholar]
- 21.Dayan AD. Albendazole, mebendazole and praziquantel. Review of non-clinical toxicity and pharmacokinetics. Acta Trop. 2003;86:141–59. doi: 10.1016/s0001-706x(03)00031-7. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Q, Gallo JM. Differential effect of sunitinib on the distribution of temozolomide in an orthotopic glioma model. Neuro Oncol. 2009;11:301–10. doi: 10.1215/15228517-2008-088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostermann S, Csajka C, Buclin T, et al. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res. 2004;10:3728–36. doi: 10.1158/1078-0432.CCR-03-0807. [DOI] [PubMed] [Google Scholar]
- 24.Luder PJ, Siffert B, Witassek F, Meister F, Bircher J. Treatment of hydatid disease with high oral doses of mebendazole. Long-term follow-up of plasma mebendazole levels and drug interactions. Eur J Clin Pharmacol. 1986;31:443–8. doi: 10.1007/BF00613522. [DOI] [PubMed] [Google Scholar]
- 25.Braithwaite PA, Roberts MS, Allan RJ, Watson TR. Clinical pharmacokinetics of high dose mebendazole in patients treated for cystic hydatid disease. Eur J Clin Pharmacol. 1982;22:161–9. doi: 10.1007/BF00542462. [DOI] [PubMed] [Google Scholar]
- 26.Messaritakis J, Psychou P, Nicolaidou P, et al. High mebendazole doses in pulmonary and hepatic hydatid disease. Arch Dis Child. 1991;66:532–3. doi: 10.1136/adc.66.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redzic Z. Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: similarities and differences. Fluids Barriers CNS. 2011;8:3. doi: 10.1186/2045-8118-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shukla S, Ohnuma S, Ambudkar SV. Improving cancer chemotherapy with modulators of ABC drug transporters. Curr Drug Targets. 2011;12:621–30. doi: 10.2174/138945011795378540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oostendorp RL, van de Steeg E, van der Kruijssen CM, et al. Organic anion-transporting polypeptide 1B1 mediates transport of Gimatecan and BNP1350 and can be inhibited by several classic ATP-binding cassette (ABC) B1 and/or ABCG2 inhibitors. Drug Metab Dispos. 2009;37:917–23. doi: 10.1124/dmd.108.024901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.