Abstract
Introduction
Transrectal prostate biopsy (TRUSbx) is the standard for the diagnosis of prostate cancer. Different bowel preparations are used for patients undergoing TRUSbx. The aim of our study was to compare two different bowel preparations for TRUSbx.
Material and methods
From May 2012 and onwards, a selected group of men undergoing TRUS 12-core prostate biopsy were enrolled into a prospective database. Patients were randomized 1:1 to receive a rectal enema (Group A) the night before the procedure or polyethylene glycol 34.8 grams/4 liters of water the day before the procedure (Group B). A VAS scale to evaluate the patients’ discomfort according to the two preparations was collected. The same antibiotic prophylaxis was performed in both groups. All complications were prospectively recorded and graded according to the Clavien Classification System (CCS).
Results
A total of 198 patients were consecutively enrolled. Mean age was 67.5 ±7.9 years, mean body mass index (BMI) was 27.1 ±4.2 Kg/m2, mean PSA value was 9.3 ±12.6 ng/ml and the mean prostatic volume was 60.6 ±29 ml. 97 patients were enrolled in Group A and 101 in Group B. Overall post-biopsy morbidity rate was 60%. No significant differences for low-grade and high-grade complications was observed between the two groups. Patients receiving the rectal enema presented with a significantly lower VAS score (3.1 ±1.1 vs. 5.9 ±1.7; p = 0.02).
Conclusions
Our study confirmed that a rectal enema should be considered as the standard bowel preparation in patients undergoing a TRUS biopsy; it is as effective as PEG and associated with less discomfort.
Keywords: prostate cancer, prostate biopsy, rectal enema, polyethylene glycol, complications
INTRODUCTION
Prostate Cancer (PCa) is a major worldwide health concern, being the second most common neoplasm and the sixth cause of cancer related death in the entire world [1]. Transrectal ultrasound-guided prostate biopsy (TRUSbx) represents the standard technique in prostate cancer diagnosis [2], although it is associated with a high risk of complications including hematospermia (37.4%), hematuria (14.5%) rectal bleeding (2.2%), infections (1%) and urinary retention (0.2%) [3].
Recently there has been a growing interest in reducing TRUSbx complications with different techniques and procedures [4–6]. Few studies have recently evaluated the possible role of bowel preparation in the prevention of TRUSbx complications [7–12]. Jeon et al. evaluated the use of bisacodyl prebiopsy rectal preparation and observed that this preparation could reduce TRUSbx infectious complications [7]. Park et al. also reported a significant reduction in infectious complications using a povidone-iodine suppository (0.3% vs. 6%) [8]. Recently, a Cochrane review concluded that enema and antibiotics together reduce the risk of bacteremia compared to antibiotics alone (RR = 0.25, 95% CI 0.08-0.75) [13].
Polyethylene glycol (PEG) is an osmotically balanced electrolyte solution that passes through the bowel without significant absorption or secretion. In a 2003 survey, the American Society of Colon and Rectal surgeons estimated that 99% of its members still prescribe some kind of MBP in order to achieve a better cleansing of the colon and rectum [14]. PEG is usually administered as a 4L solution in order to achieve a satisfactory bowel preparation for colonoscopy or rectal surgery. Theoretically, the solution passing through the rectum can mechanically remove stool and bacteria from the mucosa. So far, in combination with antibiotics, this could lead to a lower inoculation of bacteria into the prostate when the needle is inserted during TRUSbx [15]. With this knowledge in mind, we hypothesised that the PEG solution could provide a better cleansing of the rectum and so more likely reduce complications associated with the TRUSbx.
The aim of our study was to evaluate and compare, in a prospective randomized clinical trial, the role of PEG versus rectal enema in the reduction of prostate biopsy complications. As a secondary endpoint, we evaluated patient satisfaction to both methods of preparation.
MATERIAL AND METHODS
Between May 2012 and October 2013, after an internal ethical board approval, patients referred to our prostate clinic with a PSA value ≥4 ng/ml or an abnormal digital rectal examination (DRE) were scheduled for a TRUSbx after signing two written informed consents: one for the biopsy and one for the randomization. Prostate biopsy was carried out as an outpatient procedure. One week before the biopsy, patients were randomized into two different groups:
– Group A included patients who received a rectal enema the day of the biopsy.
– Group B included patients who underwent bowel preparation with 34.8 g/4L polyethylene glycol-electrolyte solution (PEG) the day before the biopsy.
According to the CONSORT guidelines [16] simple randomization was made using the sealed envelope method. A blinded operator who was not aware of the preparation performed the biopsies. Patients on anticoagulation therapy were not excluded, however, anti-platelet and anticoagulant drugs were stopped at least 1 week before the procedure. Those patients who could not interrupt anticoagulation therapy used a daily administration of subcutaneous low-molecular weight heparin (LMWH). Before the biopsy, each single patient completed a visual scale (VAS; 0 representing no discomfort and 10 representing maximum discomfort) in order to rate the discomfort associated with the bowel preparation. Antibiotic prophylaxis with Ciprofloxacin 1000 mg extended release once a day was started 24 hours before the procedure and continued for 72 hours after the procedure. Moreover, a single shot of Gentamicin 240 mg i.v. 10 min before the procedure was administered. All patients underwent 12-core TRUS-guided biopsy using a Falcon ultrasound equipment (B-K Medical, Milan, Italy) equipped with a 5–10 MHz bi-convex probe (8808 probe B-K Medical). We used a 16-gauge biopsy needle (Magnum 1000, BARD, Rome, Italy) and a dedicated spring-loaded biopsy gun (MG1522, BARD). A periprostatic anesthetic block was performed using a 20-gauge, 200-mm length spinal needle (ECOLED 2020, Rome, Italy) for the injection of 10 ml of 1% carbocaine 10 min before the biopsy [17]. Before the biopsy, patients underwent a detailed physical examination, including the measurement of height and weight. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters, squared (kg/m2). Finally, fasting (8 hr) blood samples were drawn from all patients about 2 hours before the biopsy. Serum samples were analyzed for the total PSA.
Complications evaluation
All complications within 30 days of the TRUSbx were prospectively recorded and classified according to the modified Clavien Classification System (CCS), which stratifies post-procedure complications into five grades [18]. Hematospermia lasting less than 3 days and rectal bleeding lasting less than 24h were not considered complications. Transient hematuria was defined, as reported in similar studies [19], as any hematuria persisting less than 3 days which spontaneously resolved. Hematuria lasting more than 3 days was defined as a CCS Grade 1 complication. Urinary tract infection was considered as Grade II since it required a modification in antibiotic therapy. Acute urinary retention was included as a Grade III complication since it was managed conservatively by the use of an indwelling catheter. All patients received a phone call from one of the investigators (R.L) at week one and week three after the biopsy to record eventual complications. A one-month follow-up visit was also planned.
Statistical analysis
Statistical analysis was performed using the SPSS 12.0 software. Evaluation of the data distribution showed a non-normal distribution of the study data set. Differences between groups of patients in medians for quantitative variables and difference in distributions for categorical variables were tested with Kruskal Wallis one way analysis of variance and chi-square test, respectively.
By using multiple logistic regression, the statistically significant variables as assessed in the univariate analysis were entered and investigated as predictors of complications. An alpha value of 5% was considered as the threshold for significance. Data was presented as a mean ± standard deviation (SD) and a median plus interquartile range (IQR).
Prior to the study start, a power calculation was performed based on the assumption of a reduction in the complication rate from 35% to 18% per patient. In order to identify such a variation, it was estimated that 200 evaluable patients were needed, with an 80% power using a two-tailed test at 5% significance level.
RESULTS
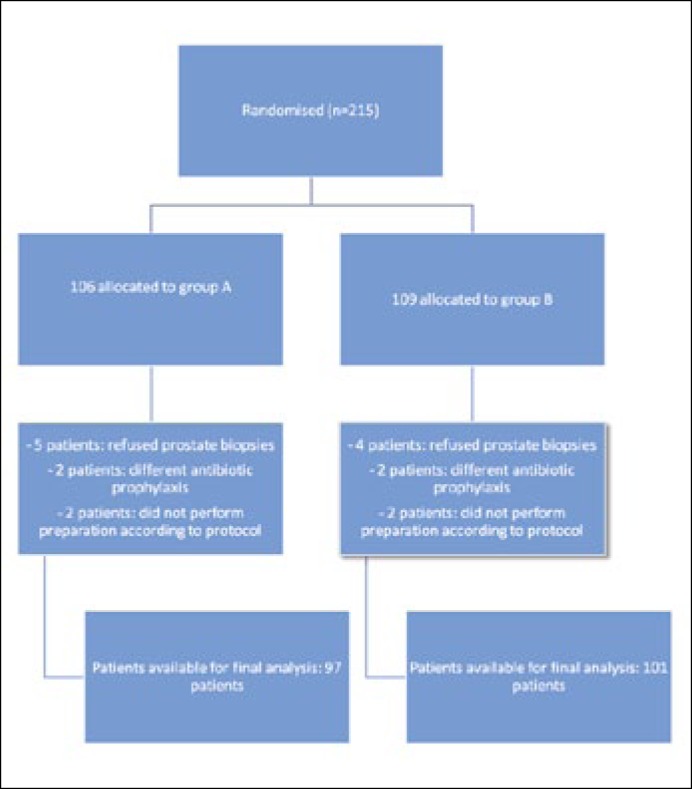
Overall 215 patients were enrolled at baseline, 198 were available. The study flow chart is shown in Figure 1. Out of these 97/198 patients (49%) underwent TRUSbx with an enema preparation while 101/198 (51%) patients underwent PEG preparation. Characteristics of the cohort are summarized in Table 1. Overall the median age was 68 (62/74) years, the median PSA was 6.8 (5.1/9.7) ng/ml and the median TRUS volume was 56.0 (35.9/82.0) ml. Overall 86/198 (43%) patients had cancer on biopsy. No differences between the groups was observed besides a significantly lower VAS score in patients receiving the rectal enema versus the PEG (3.1 ±1.1 vs. 5.9 ±1.7; p = 0.02) (Table 1).
Figure 1.
Randomization flow-chart.
Table 1.
Overall patient characteristics according to bowel preparation
| Overall | Enema Group | PEG Group | p | |
|---|---|---|---|---|
| Patients | 198 | 97/198 (49%) | 101/198 (51%) | |
| Age (years) | ||||
| Mean ±SDa | 67.9 ±7.8 | 68.5 ±7.2 | 67.2 ±8.3 | ns |
| Median (IQRb) | 68 (62/74) | 68.5 (63.3/74.0) | (67.5; 61.0/74.0) | |
| BMI (kg/m2) | ||||
| Mean ±SDa | 27.1 ±4.2 | 26.8 ±3.4 | 27.4 ±4.8 | ns |
| Median (IQRb) | 26.4 (24.7/29.0) | 26.3 (24.4/28.9) | 26.5; 24.9/29.2 | |
| PSA (ng/ml) | ||||
| Mean ±SDa | 9.3 ±12.6 | 9.9 ±3.5 | 7.4 ±4.6 | ns |
| Median (IQRb) | 6.8 (5.1/9.7) | 7.6 (5.2/11.0) | 6.4 (5.0/8.2) | |
| freePSA (ng/ml) | ||||
| Mean ±SDa | 1.3 ±1.4 | 1.4 ±1.7 | 1.2 ±1.2 | ns |
| Median (IQRb) | 1.0 (0.6/1.5) | 1.1 (0.6/1.7) | 0.9 (0.6/1.4) | |
| TRUS Volume (ml) | ||||
| Mean ±SDa | 60.6 ±29.1 | 61.2 ±30.9 | 60.1 ±27.8 | ns |
| Median (IQRb) | 56.0 (35.9 /82.0) | 56.0 (37.0/81.4) | 56.2 (35.0/84.3) | |
| Visual Scale | ||||
| Mean ±SDa | 4.2 ±1.2 | 3.1 ±1.1 | 5.9 ±1.7 | .021 |
| Median (IQRb) | 4 (2/6) | 3 (1/4) | 6 (4/6.5) | |
| Cancer | 86/198 (43%) | 44/97 (46%) | 42/101 (42%) | ns |
| Gleason Score | ||||
| 6 | 35/86 (41%) | 18/44 (41%) | 17/42 (40%) | ns |
| 7 | 26/86 (30%) | 13/44 (30%) | 13/42 (31%) | |
| 8 | 15/86 (17%) | 7/44 (16%) | 8/42 (19%) | |
| 9 | 10/86 (12%) | 6/44 (14%) | 4/42 (10%) |
SD – Standard Deviation
IQR – Interquartile Range
ns – not significant
A total of 110 complications in 198 patients were recorded prospectively. Most of them were low-grade (CCS I and II) and included hematuria during more than 3 days in 90 patients (90/110: 81%), defined as Grade I, or a change in the antibiotic regimen in 11/110 (10%) patients defined as Grade II. Overall, only 9 patients experienced high-grade complications and all of them were Grade IIIa: 7 patients presented with acute urinary retention and 2 patients with urosepsis requiring hospitalization. No Grade IV and V complications were recorded. No significant differences in terms of low or high-grade complications were observed between the two groups (Table 2).
Table 2.
Complications according to the Clavien Classification System in the two groups
| Overall | Enema Group 97/198 | PEG Group 101/198 | p | |
|---|---|---|---|---|
| Grade I | ||||
| Number | 90 | 45 | 45 | ns |
| % over patients | 90/198 (45%) | 45/97 (46%) | 45/101 (45%) | |
| % over complications | 90/110 (82%) | 45/56 (80%) | 45/54 (84%) | |
| Grade II | ||||
| Number | 11 | 6 | 5 | ns |
| % over patients | 11/198 (6%) | 6/97 (6%) | 5/101 (5%) | |
| % over complications | 11/110(10%) | 6/56 (11%) | 5/54 (9%) | |
| Grade IIIa | ||||
| Number | 9 | 5 | 4 | ns |
| % over patients | 9/198 (5%) | 5/97 (5%) | 4/101 (4%) | |
| % over complications | 9/110 (8%) | 5/56 (8%) | 4/54 (7%) | |
| Grade IIIb, IV and V | ||||
| Number | 0 | 0 | 0 | ns |
| % over patients | ||||
| % over complications | ||||
| Overall | ||||
| Number | 110 | 56 | 54 | ns |
| % over patients | 110/198 (56%) | 56/97 (58%) | 54/101 (53%) | |
| % over complications | 110/110 (100%) | 56/110 (51%) | 54/110 (49%) |
ns – not significant
DISCUSSION
TRUSbx is the most common method to evaluate and diagnose prostate cancer in patients with an elevated PSA and/or abnormal digital rectal examination. However, it is associated with a significant risk of low and high-grade complications. Loeb et al. recently summarized how TRUSbx is associated with a 10-84% rate for hematuria, 1.3-45% for rectal bleeding, 1.1-93% for hematospermia and 0-6.3% for infection requiring hospitalization [5]. Our data was in line with the available evidence, as we observed an overall complication rate of 56% although the majority of them (95%) were low-grade complications and only 5% presented as high-grade complications.
No significant differences in terms of complications were observed between the two different bowel preparations in our study. However, patients receiving a rectal enema presented with a lower VAS score when compared to patients receiving PEG preparation (5.9 ±1.7 vs. 3.1 ±1.1 p = 0.02). Our data are in line with those proposed by the Cochrane collaboration group, which recommended a rectal enema as the standard bowel preparation for patients undergoing TRUSbx [9].
Although PEG preparation is considered the standard method to prepare and reduce the risk of complications in several endoscopic and surgical procedures, data from the current literature is controversial. A recent Cochrane review concluded that there was no statistically significant evidence that patients benefit from mechanical bowel preparation (MBP), nor the use of rectal enemas for colonic surgery [13]. Bretagnol et al. evaluated, in a RCT, the role of MBP in a group of patients undergoing sphincter saving rectal surgery [20]. Their results showed that the overall and infectious morbidity rates were significantly higher in no-MBP versus MBP group, 44% vs. 27%, p = 0.018, and 34% vs. 16%, p = 0.005, respectively [20]. Moreover, Takoc et al. performed a RCT evaluating the necessity for mechanical bowel preparation before Milligan-Morgan hemorrhoidectomy in patients undergoing simple rectal enema vs. patients undergoing oral mechanical bowel preparation (MBP). They found no difference in terms of postoperative bleeding and infection [21]. Although in different procedures, our study is in line with the Cochrane review and Takoc experience, it is confirmed that MBP is not superior to rectal enema to reduce the possible complications associated with rectal procedures, such as prostate biopsies.
Ell et al. have assessed the 2 L solution of PEG plus ascorbic acid (PEG + Asc) vs. standard 4 L PEG with electrolytes (PEG + E) for bowel cleansing before colonoscopy to determine efficacy, safety and patient acceptability [22]. Patients discomfort was evaluated, as in our study, using a VAS scale and it was found that by increasing the PEG dose there was an associated higher discomfort (27.6 ±14.8 for 2l of PEG vs. 34.2 ±19.2 for 4L of PEG; p <0.02) [22]. In our experience most of the patients felt uncomfortable about the preparation, while little to no complaints were seen from patients undergoing the rectal enema.
Although it was not an objective of our study we have to also consider that the rectal enema is less expensive than the PEG preparation. In our center the median costs of a rectal enema were 12 times lower than the PEG preparation (0.50 euros vs. 6 euros). Although a cost analysis study should be conducted to obtain the appropriate results on costs, our consideration in relation to the higher cost associated with PEG preparation further supports the rectal preparation with an enema instead of PEG for bowel preparation in patients undergoing TRUSbx. Rectal enema is less expensive, more acceptable for the patients and as effective as PEG in patients scheduled for TRUSbx.
Despite many efforts in defining the role of MBP in surgery, current literature lacks high-level evidence. The most recent reviews and meta-analysis cannot conclude in favor nor against this 30 year old routine [23]. Dahabreh IJ et al. recently published an executive summary on oral mechanical bowel preparation for colorectal surgery including 65 publications [24]. Their results concluded that the evidence on the use of MBP or not is weak and future studies including pooled reanalysis of existing data and new comparative studies are needed [24]. Duncan et al. analyzed also the status of bowel preparation, in terms of infection all the trials included showed no statistical difference between MPB groups and no MBP groups [25]. Nonetheless, Kumar et al. analyzed 14 RCT on MBP in their review on bowel preparation before elective surgery [15]. In terms of complications, 13/14 trials found no difference between patients with or without MBP. However, at present no national society has publicly endorsed the abandonment of MBP in elective colorectal surgery. Moreover, only recently, the role of MBP in urologic surgery [26] and procedures has been investigated. Our study adds more evidence to the trend in reducing MBP in surgery and procedures. Although, whether historical doctrine or unsubstantiated dogma, routine MBP may require further debate before it is relegated to an antiquated practice.
We have to acknowledge some limitations encountered in our study. Our study was a single center trial and further trials are probably needed to confirm our experience. Another possible limitation of this study was the low rate of infectious complications, which clearly would have warranted more patients for the analysis to investigate possible differences between the two groups of patients. The lack of a control group could also be considered another limitation, however, our study was designed to evaluate the overall rate of complications and patients discomfort to both preparations. Considering our results and the patients discomfort with the PEG preparation we decided not to continue our research in this field and to abandon the MBP in patients scheduled for TRUSbx. Notwithstanding all this limitations, our study firstly evaluated and compared a MBP vs. the standard (rectal enema) as a possible alternative rectal preparation in patients undergoing TRUSbx.
CONCLUSIONS
In our single center randomized clinical trial, there were no significant differences in terms of complications observed between the two different bowel preparations. PEG preparation was considered less comfortable by our patients. According to our results, we confirmed the use of rectal enema as the standard method for rectal preparation in patients scheduled for prostatic biopsy.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rate. Eur Urol. 2012;61:1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 2.Persson G, Danielsson M, Rosén M, et al. Health in Sweden: The National Public Health Report 2005. Scand J Public Health Suppl. 2006;67:3–10. doi: 10.1080/14034950600677360. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich A, Bastian PJ, Bellmunt J, et al. Guidelines on Prostate Cancer. EAU Guidelines. Eur Urol. 2014;65:124–137. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 4.Ehdaie B, Vertosick E, Spaliviero M, et al. The Impact of Repeat Biopsies on Infectious Complications in Men with Prostate Cancer on Active Surveillance. J Urol. 2014;191:660–664. doi: 10.1016/j.juro.2013.08.088. [DOI] [PubMed] [Google Scholar]
- 5.Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011;186:1830–1834. doi: 10.1016/j.juro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmignani L, Picozzi S, Spinelli M, et al. Bacterial sepsis following prostatic biopsy. IntUrol Nephrol. 2012;44:1055–1063. doi: 10.1007/s11255-012-0145-9. [DOI] [PubMed] [Google Scholar]
- 7.Jeon SS, Woo SH, Hyun JH, Choi HY, Shai SE. Bisacodyl rectal preparation can decrease infectious complications of transrectal ultrasound-guided prostate biopsy. Urology. 2003;62:461–466. doi: 10.1016/s0090-4295(03)00470-9. [DOI] [PubMed] [Google Scholar]
- 8.Park DS, Oh JJ, Lee JH, Jang WK, Hong YK, Hong SK. Simple use of the suppository type povidone-iodine can prevent infectious complications in transrectal ultrasound-guided prostate biopsy. Adv Urol. 2009:750598. doi: 10.1155/2009/750598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zani EL, Clark OA, Rodrigues Netto N., Jr Antibiotic prophylaxis for transrectal prostate biopsy. Cochrane Database Syst Rev. 2011;5:CD006576. doi: 10.1002/14651858.CD006576.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Gil-Vernet Sedo JM, Alvarez-Vijande Garcia R. Effect of intrarectal povidone-iodine in the incidence of infectious complications after transrectal prostatic biopsy. Arch EspUrol. 2012;65:463–466. [PubMed] [Google Scholar]
- 11.Abughosh Z, Margolick J, Goldenberg SL, et al. A prospective randomized trial of povidone-iodine prophylactic cleansing of the rectum before transrectal ultrasound guided prostate biopsy. J Urol. 2013;189:1326–1331. doi: 10.1016/j.juro.2012.09.121. [DOI] [PubMed] [Google Scholar]
- 12.Allard P, Bruce W, Janelle D, et al. Use of Eductyl® suppository for rectal preparation before prostatebiopsy: an observational survey. Prog Urol. 2012;22:166–171. doi: 10.1016/j.purol.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Güenaga KF, Matos D, Wille-Jørgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev. 2011;7:CD001544. doi: 10.1002/14651858.CD001544. [DOI] [PubMed] [Google Scholar]
- 14.Zmora O, Wexner SD, Hajjar L, et al. Trends in preparation for colorectal surgery: survey of the members of the American Society of Colon and Rectal Surgeons. Am Surg. 2003;69:150–154. [PubMed] [Google Scholar]
- 15.Kumar AS, Kelleher DC, Sigle GW. Bowel preparation before elective surgery. Clin Colon Rectal Surg. 2013;26:146–152. doi: 10.1055/s-0033-1351129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trucchi A, De Nunzio C, Mariani S, Palleschi G, Miano L, Tubaro A. Local anesthesia reduces pain associated with transrectal prostatic biopsy. A prospective randomized study. Urol Int. 2005;74:209–213. doi: 10.1159/000083550. [DOI] [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patiens and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandal S, Sankhwar SN, Kathpalia R, et al. Grading complications after trans urethral resection of the prostate using Clavien Classification System and predicting complications using the Charlson comorbidity index. Int Urol Nephrol. 2013;45:347–354. doi: 10.1007/s11255-013-0399-x. [DOI] [PubMed] [Google Scholar]
- 20.Bretagnol F, Panis Y, Rullier E, et al. Rectal cancer surgery with or without bowel preparation: The French GRECCAR III multicenter single-blinded randomized trial. Ann Surg. 2010;252:863–868. doi: 10.1097/SLA.0b013e3181fd8ea9. [DOI] [PubMed] [Google Scholar]
- 21.Tokaç M, Bozkurt B, Gürkan Dumlu E, et al. Evaluation of necessity for mechanical bowel preparation before Milligan-Morgan hemorrhoidectomy: a randomized prospective clinical study. Minerva Chir. 2013;68:393–399. [PubMed] [Google Scholar]
- 22.Ell C, Fischbach W, Bronisch HJ, et al. Randomized trial of low-volume PEG solution versus standard PEG + electrolytes for bowel cleansing before colonoscopy. Am J Gastroenterol. 2008;10:883–893. doi: 10.1111/j.1572-0241.2007.01708.x. [DOI] [PubMed] [Google Scholar]
- 23.Hughes ESR. Asepsis in large-bowel surgery. Ann R Coll Surg Engl. 1972;51:347–356. [PMC free article] [PubMed] [Google Scholar]
- 24.Dahabreh IJ, Steele DW, Shah N, Trikalinos TA. Oral Mechanical Bowel Preparation for Colorectal Surgery [Internet]; Rockville (MD): Agency for Healthcare Research and Quality (US); 2014. Report No.: 14-EHC018-EF.PMID= 24921111. [PubMed] [Google Scholar]
- 25.Duncan JE, Quietmeyer CM. Bowel preparation: current status. Clin Colon Rectal Surg. 2009;22:14–20. doi: 10.1055/s-0029-1202881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cerantola Y, Valerio M, Persson B, et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: enhanced Recovery After Surgery (ERAS®) society recommendations. Clin Nutr. 2013;32:879–887. doi: 10.1016/j.clnu.2013.09.014. [DOI] [PubMed] [Google Scholar]