Figure 2. Rad50 hook mutations destabilize hook dimerization.
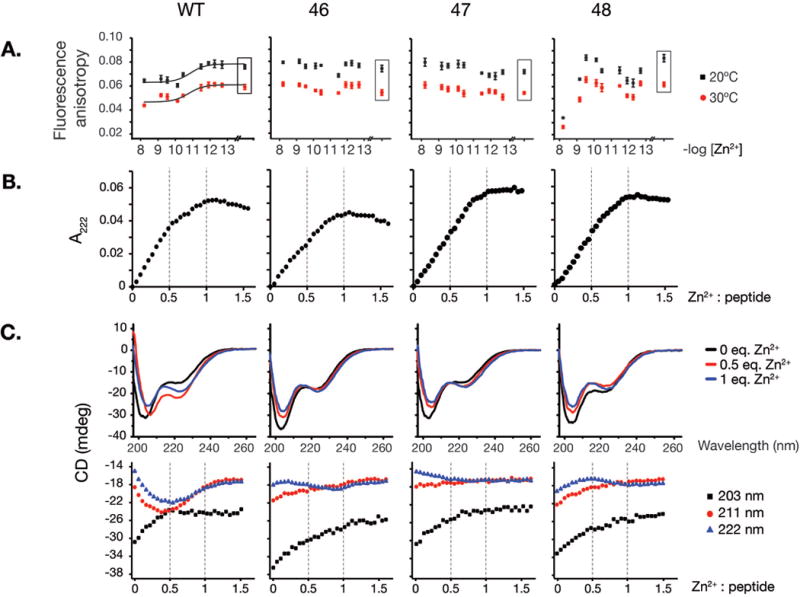
A. Homo-FRET of 5-carboxyfluorescein labeled 68 amino acid long hook peptides. Fluorescence anisotropy values for wild-type and 46, 47, 48 hook mutant peptides incubated in free Zn2+-controlled metal buffers providing various free zinc concentrations (−log[Zn2+]) are plotted. Data points shown in a box reflects buffer without Zn2+, containing chelator only (1 mM EDTA). Data were fitted to Hill’s equation with cooperativity coefficient fixed at 1.0. B. Titration of 41-mer WT and Rad50hook peptides with Zn2+ monitored by spectroscopy at 222 nm. Formation of coordinate bonds is observed as CysS-> Zn LMCT bands that can be monitored in the UV region. C. Circular dichroism (CD) spectra of wild-type and Rad50-46 hook mutant 41-mer unlabeled peptides. CD spectra of peptides with 0 (apo-peptide), 2.5 μM (0.5 equivalent of Zn2+) and 5 μM Zn2+ (1 eq. of Zn2+) at 203, 211 and 222 nm are given.
