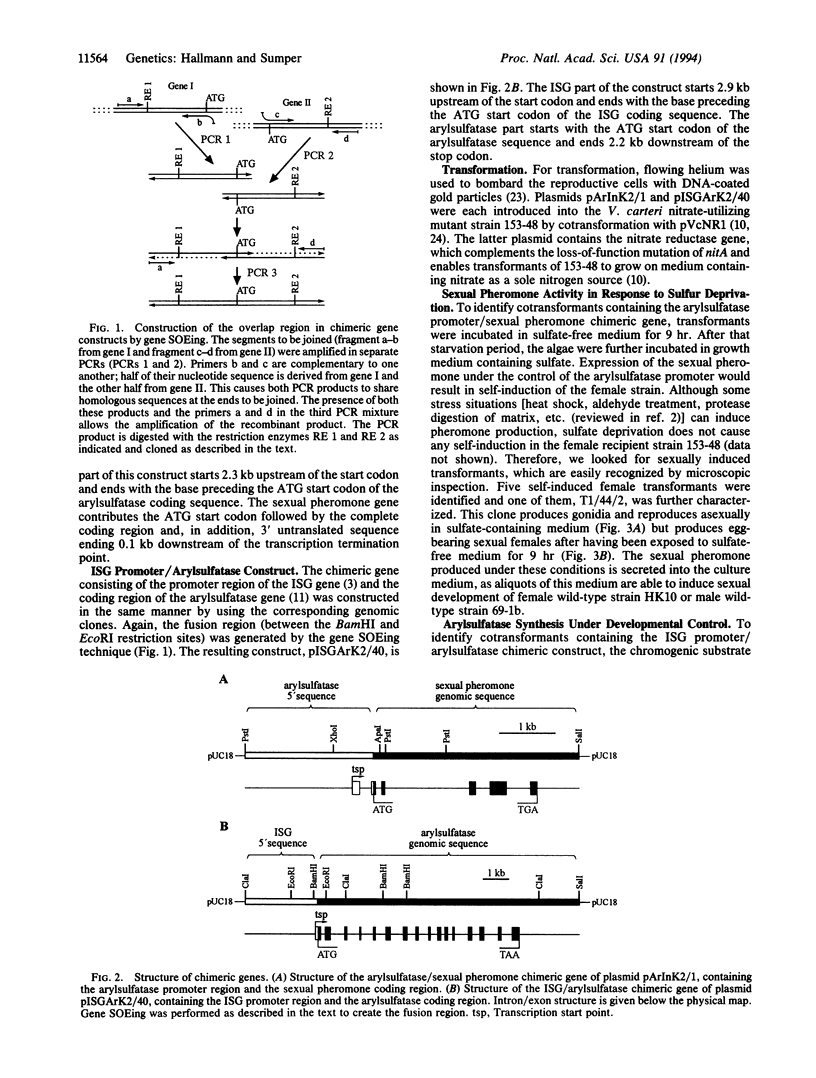
Abstract
The multicellular alga Volvox is an attractive model for the study of developmental processes. With the recent report of successful transformation, regulated promoters as well as reporter genes working in this organism are now required. The Volvox genes encoding arylsulfatase and the extracellular glycoprotein ISG are strictly regulated. The former is transcribed only under conditions of sulfur starvation, whereas the latter operates under extreme developmental control--i.e., it is transcribed for only a few minutes in Volvox embryos at the stage of embryonic inversion. The gene encoding the sexual pheromone of Volvox carteri was placed under the control of the arylsulfatase promoter. In response to sulfur deprivation, V. carteri transformed by this construct synthesized and secreted biologically active pheromone. In addition, the gene encoding Volvox arylsulfatase was placed under the control of the ISG promoter. Transformed algae synthesized arylsulfatase mRNA only during embryonic inversion. These experiments demonstrate the usefulness of both the arylsulfatase and the sexual pheromone reporter genes. In addition, the highly regulated arylsulfatase promoter allows the construction of inducible expression vectors for cloned genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. R., Stamer K. A., Miller J. K., McNally J. G., Kirk M. M., Kirk D. L. Patterns of organellar and nuclear inheritance among progeny of two geographically isolated strains of Volvox carteri. Curr Genet. 1990 Aug;18(2):141–153. doi: 10.1007/BF00312602. [DOI] [PubMed] [Google Scholar]
- Ertl H., Hallmann A., Wenzl S., Sumper M. A novel extensin that may organize extracellular matrix biogenesis in Volvox carteri. EMBO J. 1992 Jun;11(6):2055–2062. doi: 10.1002/j.1460-2075.1992.tb05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber H., Goetinck S. D., Kirk D. L., Schmitt R. The nitrate reductase-encoding gene of Volvox carteri: map location, sequence and induction kinetics. Gene. 1992 Oct 12;120(1):75–83. doi: 10.1016/0378-1119(92)90011-d. [DOI] [PubMed] [Google Scholar]
- Hallmann A., Sumper M. An inducible arylsulfatase of Volvox carteri with properties suitable for a reporter-gene system. Purification, characterization and molecular cloning. Eur J Biochem. 1994 Apr 1;221(1):143–150. doi: 10.1111/j.1432-1033.1994.tb18723.x. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989 Apr 15;77(1):61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Huskey R. J., Griffin B. E., Cecil P. O., Callahan A. M. A Preliminary Genetic Investigation of VOLVOX CARTERI. Genetics. 1979 Feb;91(2):229–244. doi: 10.1093/genetics/91.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindle K. L., Schnell R. A., Fernández E., Lefebvre P. A. Stable nuclear transformation of Chlamydomonas using the Chlamydomonas gene for nitrate reductase. J Cell Biol. 1989 Dec;109(6 Pt 1):2589–2601. doi: 10.1083/jcb.109.6.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk D. L., Harper J. F. Genetic, biochemical, and molecular approaches to Volvox development and evolution. Int Rev Cytol. 1986;99:217–293. doi: 10.1016/s0074-7696(08)61428-x. [DOI] [PubMed] [Google Scholar]
- Mages H. W., Tschochner H., Sumper M. The sexual inducer of Volvox carteri. Primary structure deduced from cDNA sequence. FEBS Lett. 1988 Jul 18;234(2):407–410. doi: 10.1016/0014-5793(88)80126-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiedlmeier B., Schmitt R., Müller W., Kirk M. M., Gruber H., Mages W., Kirk D. L. Nuclear transformation of Volvox carteri. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5080–5084. doi: 10.1073/pnas.91.11.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt R., Fabry S., Kirk D. L. In search of molecular origins of cellular differentiation in Volvox and its relatives. Int Rev Cytol. 1992;139:189–265. doi: 10.1016/s0074-7696(08)61413-8. [DOI] [PubMed] [Google Scholar]
- Starr R. C., Jaenicke L. Purification and characterization of the hormone initiating sexual morphogenesis in Volvox carteri f. nagariensis Iyengar. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1050–1054. doi: 10.1073/pnas.71.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
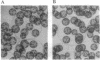
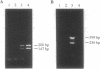
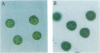
- Sumper M., Berg E., Wenzl S., Godl K. How a sex pheromone might act at a concentration below 10(-16) M. EMBO J. 1993 Mar;12(3):831–836. doi: 10.1002/j.1460-2075.1993.tb05723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y., Dotson M., Keen N. T. Plant transformation: a simple particle bombardment device based on flowing helium. Plant Mol Biol. 1992 Feb;18(4):835–839. doi: 10.1007/BF00020031. [DOI] [PubMed] [Google Scholar]
- Tschochner H., Lottspeich F., Sumper M. The sexual inducer of Volvox carteri: purification, chemical characterization and identification of its gene. EMBO J. 1987 Aug;6(8):2203–2207. doi: 10.1002/j.1460-2075.1987.tb02491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]