Fig. 1.
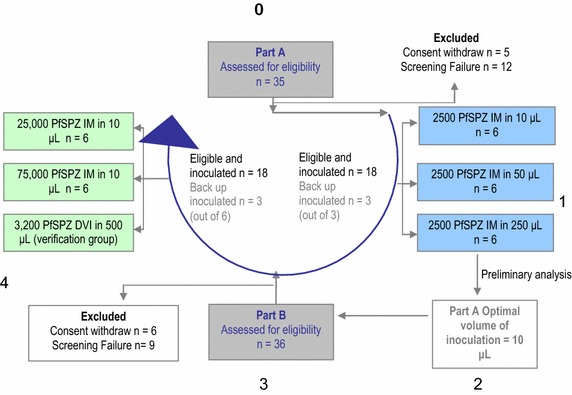
Flow chart of volunteer recruitment. This trial was divided in two parts. In part A the impact of volume of inoculation on infectivity rate and pre-patent period was tested by injecting the same dose of P. falciparum sporozoites (PfSPZ) in three different injection volumes. In part B, the volume that resulted in the highest infectivity rate was used for the formulation of two increased IM doses. A DVI group was included in part B to independently corroborate the results of the trial in Tübingen [18]. IM intramuscular, DVI direct venous inoculation, back up refers to the extra volunteers who were enrolled in each part of the study. Group 1: 2,500 PfSPZ in 10 μL; group 2: 2,500 PfSPZ in 50 μL; group 3: 2,500 PfSPZ in 250 μL; group 4: 3,200 PfSPZ in 500 μL; group 5: 25,000 PfSPZ in 10 μL; group 6: 75,000 PfSPZ in 10 μL.
