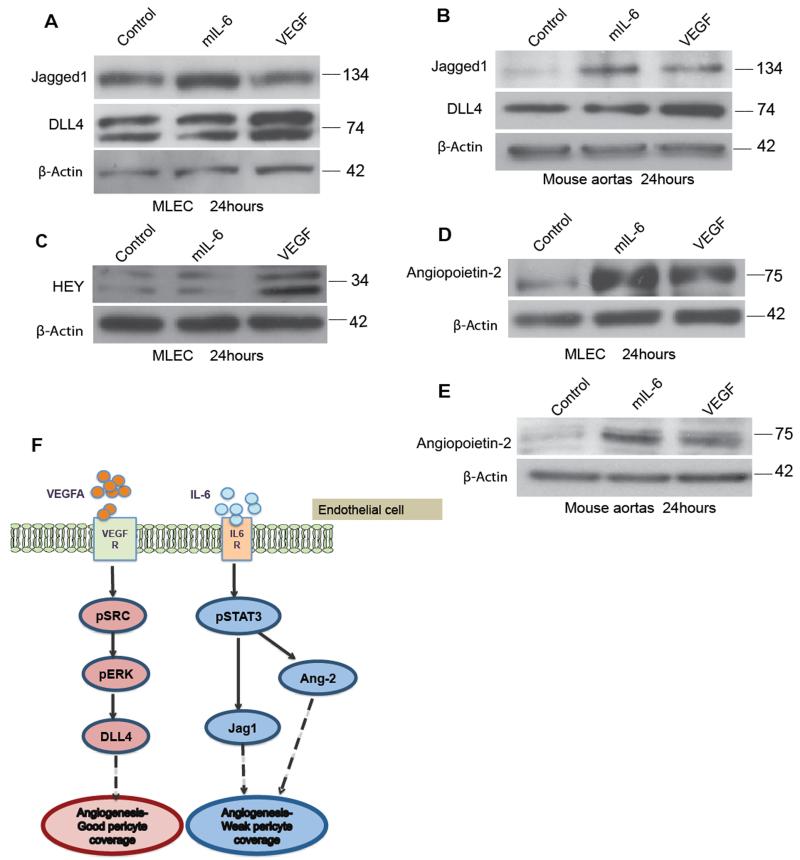
Figure 4. Differential regulation of VEGF and IL-6 mediated Notch ligands and Ang2 may explain defective pericyte coverage.
2×105 MLEC cells were plated and treated with control (PBS), VEGF (30ng/ml) or mIL-6 (30ng/ml) for 24 hours. A. Western blot analysis of Jagged1 and DLL4 expression on MLEC cells. B. The induction of Jagged1 by IL-6 and DLL4 by VEGF is further confirmed in the protein analysis of aortas treated with IL-6 or VEGF. C. Hey upregulation in the VEGF treated MLEC confirms the positive VEGF induced Notch-DLL4 interaction in the MLEC. D. Western blot analysis of Ang2 in the MLEC treated with VEGF or IL-6 shows similar upregulation of Ang2 in the MLEC treated with IL-6 compared to VEGF E. Ang2 expression in protein isolated from three aortas per group from wild-type C57BL/6 mice (8–12 weeks) and treated with either VEGF (30ng/ml) or mouse IL-6 (30ng/ml) for 24hours F. Model for mechanism of action of VEGF or IL-6 on endothelial cells. VEGF binding to VEGF receptor leads to upregulation of DLL4 resulting in angiogenesis and maturation of vessels. However, IL-6 forms a complex with IL-6 receptors to activate Jagged1 and Ang2 resulting in angiogenesis with weak or defective pericyte coverage.