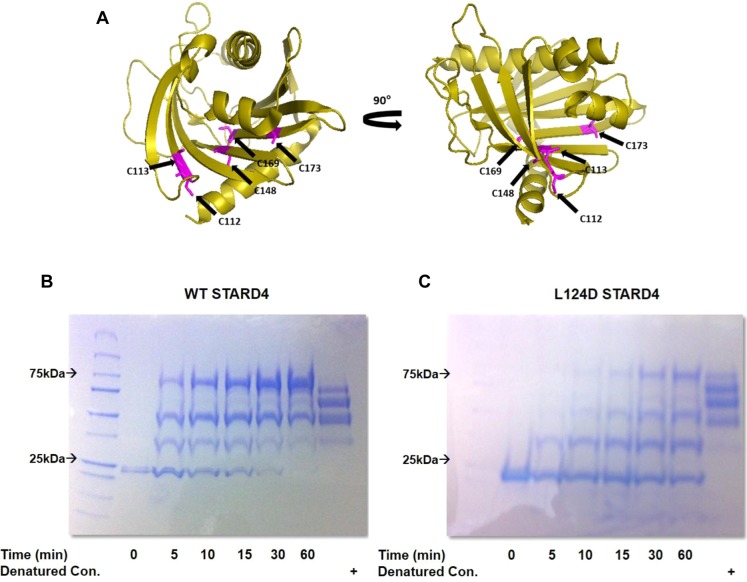
Figure 7.
Mutation of the Ω1 loop in STARD4 stabilizes a closed conformation. (A) Ribbon representation of STARD4 with the five cysteines colored magenta. (B) SDS–PAGE gel of the maleimide–PEG 5 kDa reaction with wild-type and L124D STARD4. Molecular weight shifts are the result of the nonhydrolyzable linkage between maleimide and the STARD4 thiol side chain. Reactions were conducted at 37 °C and quenched with β-mercaptoethanol prior to denaturing and electrophoresis. As a positive control, wild-type and L124D STARD4 were denatured with SDS and incubated for 60 min with maleimide-PEG 10 kDa.