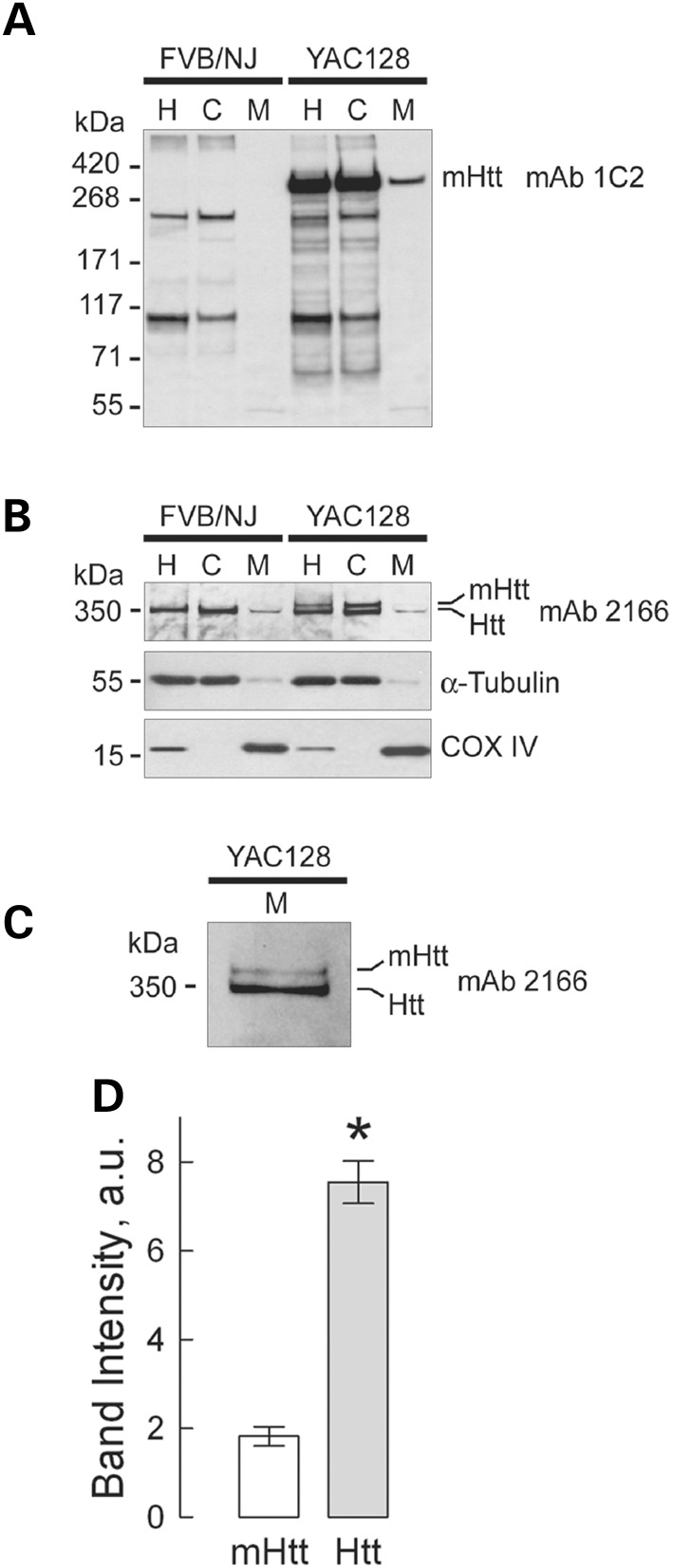
Figure 2.
Detection of wild-type mouse huntingtin (Htt) and mutant human huntingtin (mHtt) in homogenates, and cytosolic and synaptic mitochondrial fractions from 2-month-old YAC128 and WT mice. In (A) mHtt was detected with anti-polyQ 1C2 antibody as a single band exclusively in samples from YAC128 mice. The bands with lower molecular weights (∼200 kDa and 100 kDa) are present in both WT and YAC128 samples and most likely represent a result of non-specific cross-reaction with unidentified polyglutamine-containing proteins. In (B) Htt and mHtt were detected using western blotting with 2166 antibody, which recognizes both Htt and mHtt. In homogenates and cytosolic fractions from WT and YAC128 mice, a 350 kDa band, belonging to wild-type Htt, was detected, whereas two bands, most likely representing both Htt and mHtt, were detected in homogenates and cytosolic fractions from YAC128 mice. Tubulin and COX IV were used as cytosolic and mitochondrial markers, respectively. In mitochondrial fractions from both WT and YAC128 mice, faint bands corresponding to Htt were detected, whereas amounts of mHtt attached to mitochondria were too low to be reliably detected with 2166 antibody. Only after an increase in protein loading (50 µg/lane) and in exposure time of the film, did mHtt band become evident (C). In A–C: H, homogenate; C, cytosolic fraction; M, mitochondrial fraction. In (D), the results of densitometry of mHtt and Htt bands obtained with 2166 antibody applied to mitochondrial samples. Data are mean ± SEM, N = 7, *P < 0.01 comparing band intensity for mHtt and wild-type Htt.