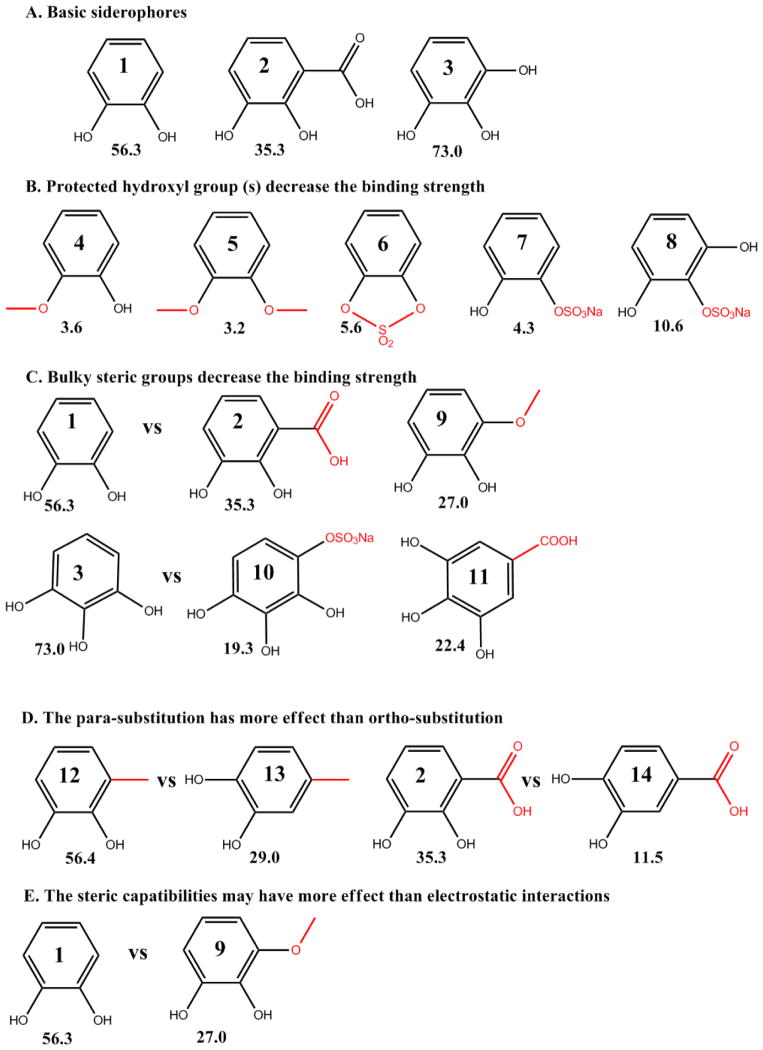
Figure 6.
The binding strength of catechol and its derivatives (% retention of 55Fe3+), was tested with NGAL-Siderocalin, 55Fe3+ and 10 μM compounds (see Materials and Methods). Compounds 1: catechol, 2: 2,3-DHBA, 3: pyrogallol, 4: guaiacol, 5: 1,2-dimethoxybenzene, 6: catechol cyclic sulfate, 7: catechol sulfate sodium, 8: pyrogallol-2-Sulfate, 9: 3-methoxycatechol, 10: pyrogallol-4-Sulfate, 11: gallic acid, 12: 3-methylcatechol, 13: 4-methylcatechol, 14: 3,4-dihydroxybenzoic Acid (A) Basic siderophores (B) Protected hydroxyl group(s) dramatically decrease binding strength (C) Bulky steric groups decrease the binding strength (D) The para-position substitution has a greater effect than the ortho-position substitute (E) The steric compatibilities may have greater importance than electrostatic interactions. [14]