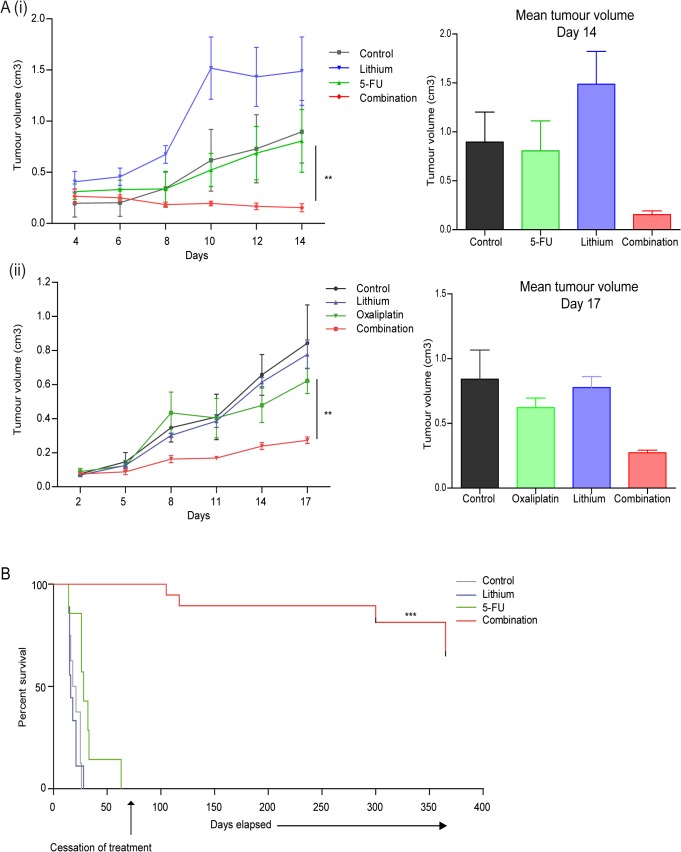
Fig 5. In vivo implementation of combination therapy in pre-clinical CT26 colorectal carcinoma model.
To assess the effects of combination therapy on tumor volume and survival (A) (i) Colorectal carcinoma cells (CT26) (1 x 106) were injected subcutaneously into the right flank of female balb/c mice (n = 3 per group). When mean tumor diameters were 0.5 cm +/- 0.02cm (~14 days post injection of tumor cells) all animals received an intratumoral injection every three days of either PBS (control), 5-fluorouracil (5-FU) (20 mg/kg), lithium chloride (200 mg/kg) or combinations of 5-FU and lithium chloride for two weeks. Tumor size was monitored by alternate day measurements in two dimensions, using verniers callipers, with first measurement recorded four days after initial treatment. Tumor volume was calculated according to the formula V = ab2ᴨ/6, where ‘a’ is the longest diameter of the tumor and ‘b’ is the longest diameter perpendicular to diameter ‘a’. (ii) CT26 cells (1 x 106) were injected and tumors allowed to develop as above (n = 4 per group). To assess effects of systemically delivered combination therapy on primary tumors all treated and control groups received intraperitoneal injection every three days, of either PBS (control), oxaliplatin (10 mg/kg), lithium chloride (200 mg/kg) or combinations of oxaliplatin and lithium chloride for up to three weeks. Tumor size was assessed every three days, with the first measurement recorded two days after initial treatment. Tumor volume was calculated as above. Asterisks indicate a significant difference in the mean tumor volume of combination treated animals compared to single agent treated tumors (5-FU or oxaliplatin) (** p < 0.01) (unpaired t-test). (B) To assess the antitumor effect of combination therapy on survival, CT26 tumors were induced as above in balb/c mice (n = 8 per group) and once tumors were established all treated and control groups received an intratumoral injection every three days with PBS (control), lithium chloride (200mg/kg), 5-FU (20mg/kg) and a combination of both lithium chloride and 5-FU (200mg/kg, 20mg/kg). When tumor volume reached ~ 1.5 cm3 (no greater than 1.5 cm in diameter) animals were euthanized. The median survival for control treated animals was 19.5 days, lithium treated– 16 days, 5-FU treated– 28 days. Median survival of combination treated group is undefined due to long term survival of more than half the group. Treatment of the remaining combination treated animals was maintained until day 75, following which all treatments ceased. One year after treatment began these animals remained tumor free. Asterisks indicate a significant difference in the overall median survival of combination treated (5-FU & lithium chloride) animals compared to single agent treated animals (5-FU) (*** p < 0.0001). Statistical analysis was carried out using the log-rank test.