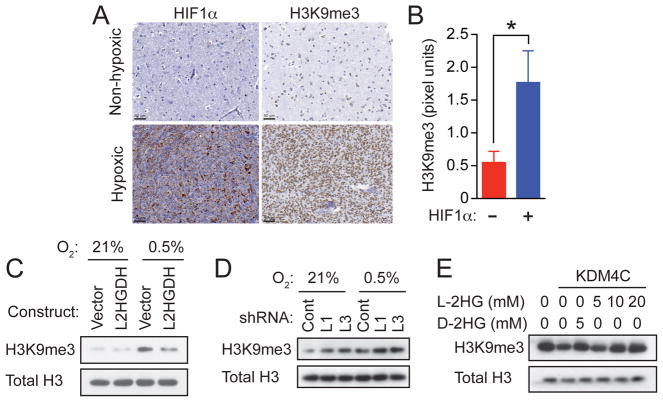
Figure 4. L-2HG is Necessary and Sufficient for Enhanced Trimethylation of Histone 3 Lysine 9 in Response to Hypoxia.
(A) HIF1α expression and H3K9me3 levels were assessed by immunohistochemistry in human glioblastomas specimens with (n=26) or without (n=21) hypoxic regions captured in the biopsy. Scale bars are shown in the bottom left of each panel.
(B) Quantification of H3K9me3 staining intensity (pixel units) within biopsies not expressing (-) or expressing (+) HIF1α. Values represent mean +/- SD; *p=0.01 determined by unpaired two-tailed Student’s t test.
(C) SF188 glioblastoma cells stably infected with empty lentiviral vector (Vector) or lentiviral vector encoding L2HGDH were cultured for 48 hr in 21% or 0.5% O2, followed by histone extraction and assessment of H3K9me3 by Western blot.
(D) SF188 glioblastoma cells stably infected with lentiviruses expressing non-targeting shRNA (Cont) or shRNAs targeting L2HGDH (L1, L3) were cultured in 21% or 0.5% O2, followed by histone extraction and assessment of H3K9me3 by Western blot.
(E) Bulk histones were incubated with purified human KDM4C in a reaction mixture with α-KG (1mM), as well as the indicated concentrations of L-2HG or D-2HG. H3K9me3 was assessed by Western blot with total H3 used as loading control. Panels in (C), (D), and (E) show representative data from 1 of 3 independent experiments. See also Figure S4.