Figure 3. The intrinsic chemical exchange map of yAAC3.
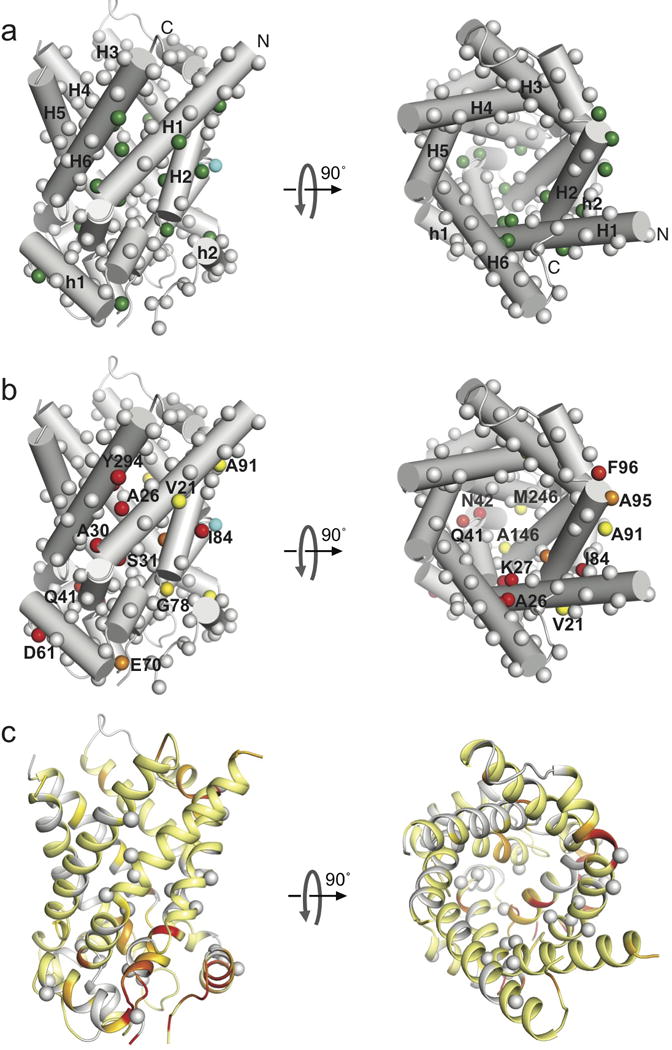
a. Cylinder representation of yAAC3 (PDB ID: 4C9Q28) showing backbone amide 15N nuclei of free yAAC3 with significant chemical exchange (green spheres). These residues all show kex ~ 870 ± 200 s−1. Grey spheres represent residues with flat relaxation dispersion curves. Spheres in cyan indicate severely exchange-broadened residues.
b. The same representation as in (a) showing residue-specific chemical shift differences between the ground and excited states, Δω. The spheres are colored according to the Δω value using a linear gradient from yellow (0.5 ppm) to red (4 ppm).
c. Residue-specific chemical shift changes (ΔΩ) induced by the addition of 10 mM ADP mapped to the yAAC3 structure in the same orientation as in (a) and (b). The ΔΩ are shown with a linear color spectrum scale from 0.01 ppm (yellow) to 0.03 ppm (red). The residues having strong relaxation dispersion are indicated as grey spheres, for showing the difference between chemical exchange and ADP-induced chemical shift changes.
