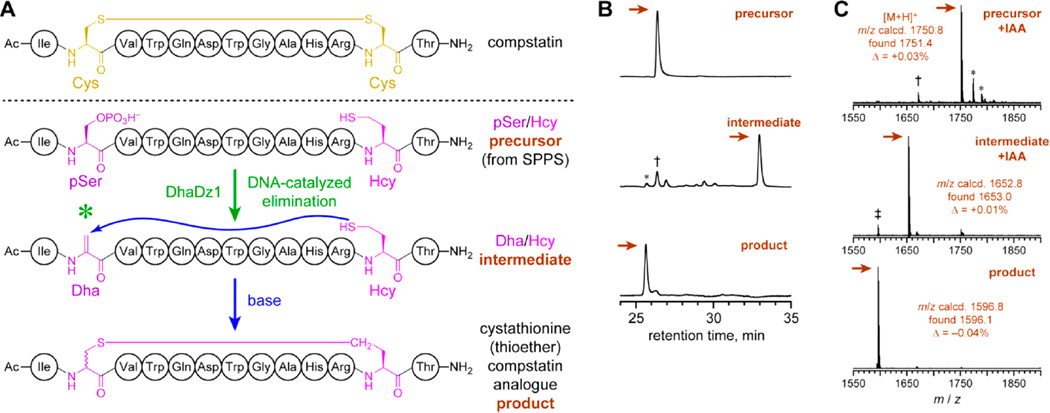
Figure 5.
Application of the DhaDz1 pSer lyase deoxyribozyme for synthesis of a more stable compstatin analogue. (A) Compstatin, and its stable cystathionine (thioether) analogue as prepared using DhaDz1. One of the two possible pSer/Hcy combinations is shown. (B) HPLC analysis of the reaction process. The three traces show (top to bottom) the HPLC-purified linear precursor pSer/Hcy peptide, the crude linear intermediate Dha/Hcy peptide after treatment of the precursor with DhaDz1, and the crude cyclic product (compstatin thioether analogue) after subsequent base treatment of the HPLC-purified intermediate Dha/Hcy peptide. Single-turnover conditions for Dha formation: 100 µM DhaDz1, 125 µM untethered DNA anchor oligo, 50 µM precursor peptide, 70 mM HEPES, pH 7.5, 4 mM ZnCl2 (optimized), 20 mM MnCl2, 40 mM MgCl2, 150 mM NaCl, 37 °C, 96 h. Conditions for cyclization: 100 mM Tris, pH 8.0, 25 °C, 4 h (shorter times not attempted). The two-step yield was 70%. For the intermediate, the asterisk denotes autocyclization product, the dagger denotes unreacted precursor, and unmarked peaks are side products not specifically identified. (C) MALDI mass spectrometry of HPLC-purified precursor, intermediate, and product, each after iodoacetamide (IAA) treatment to modify any free thiol groups (+57; one free thiol in each of precursor and intermediate; no free thiol in product).28 For the precursor, the asterisks denote [M + Na]+ and [M + K]+, and the dagger denotes hydrolysis of pSer. For the intermediate, the double dagger denotes autocyclization product.