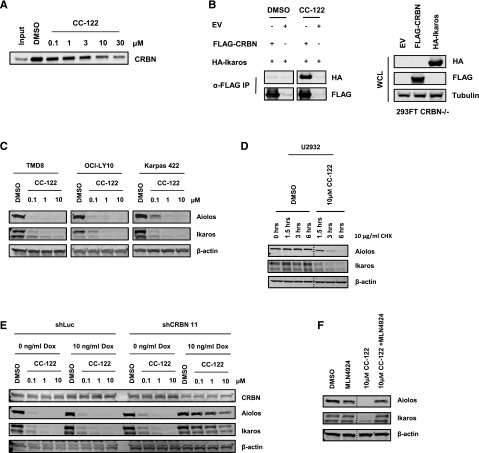
Figure 2.
Aiolos and Ikaros are CRL4CRBN-dependent substrates of CC-122. (A) U266 cell lysates incubated with thalidomide analog bound FG affinity beads were treated with either DMSO or multiple concentrations of CC-122. Two hours later, complexes were washed, and bound protein was detected by immunoblot analysis for CRBN. (B) 293FT CRBN−/− cells were transiently transfected with pcDNA3.1, pcDNA3.1-FLAG-CRBN, or pcDNA3.1 HA-IKZF1, respectively (left panel). Whole cell extracts were then mixed with FLAG M2 beads (Sigma) for 24 hours at 4°C. Where indicated, CC-122 (20 μM) or DMSO were added into the binding reaction 24 hours before the complexes were washed, and bound protein was detected by immunoblot analysis. Cell lysates were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and levels of hemagglutinin (HA), FLAG, and tubulin were assessed (right panel). (C) DLBCL cells were treated with DMSO or CC-122 (0.1-10 μM) for 12 hours. Cell lysates were separated by SDS-PAGE, and levels of Aiolos, Ikaros, and β-actin were assessed by immunoblot analysis and quantification. (D) U2932 cells were pulsed with 10 μg/mL cycloheximide (CHX), then treated with DMSO or CC-122 (10 μM) for indicated time points. Cell lysates were separated by SDS-PAGE, and levels of Aiolos, Ikaros, and β-actin were assessed. (E) OCI-LY10 cells expressing shRNA targeting luciferase or CRBN were treated with either 0 or 10 ng/mL Dox for 48 hours. After 48 hours, cells were treated with DMSO or indicated doses of CC-122 for an additional 12 hours. Cell lysates were separated by SDS-PAGE, and levels of CRBN, Aiolos, and β-actin were assessed. (F) TMD8 cells were pretreated with MLN4924 (10 μM) for 1 hour, then treated with DMSO or CC-122 (10 μM) for 6 hours. Cell lysates were separated by SDS-PAGE, and levels of Aiolos, Ikaros, and β-actin were assessed as before.