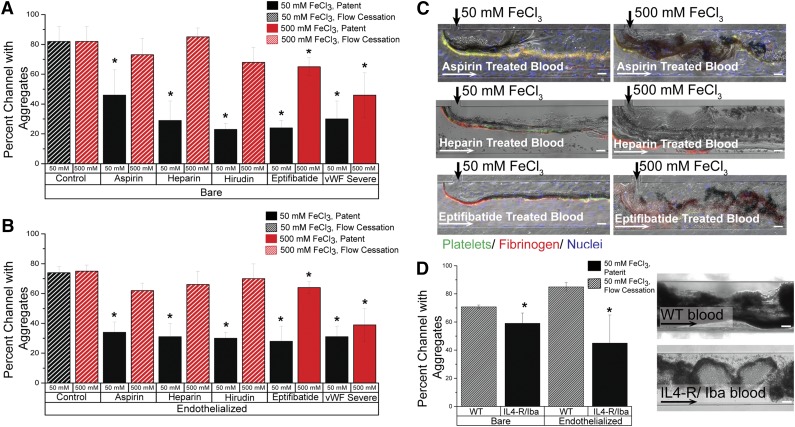
Figure 6.
Aggregation of blood in altered clotting environments. (A) In bare microfluidics, all drugs and phenotypic abnormalities lead to significant decreases in channel occlusion at 50 mM (P < .05) compared with the control solution, but 500 mM FeCl3 overcomes the protective effect of aspirin, heparin, and hirudin. Solid bars represent patent channels while the slant pattern represents flow cessation. Significance is denoted by an asterisk (*), and was measured by a 2-tailed, Student t test between the treatment condition and its paired control (eg, 50 mM control and 50 mM aspirin). There was not a significant difference in occlusion between untreated control blood exposed to 50 mM and that exposed to 500 mM. (B) In endothelialized microfluidics, drugs and phenotypic abnormalities lead to significant decreases in occlusion at 50 mM (P < .5), but 500 mM FeCl3 overcomes the protective effect. Solid bars represent patent channels whereas the slant pattern represents flow cessation. Significance is denoted by an asterisk (*), and was measured by a 2-tailed, Student t test between the treatment condition and its paired control (eg, 50 mM control and 50 mM aspirin). There was not a significant difference in occlusion between untreated control blood exposed to 50 mM and that exposed to 500 mM. (C) Representative images from drug treatments in bare and endothelialized microfluidics, at 50 mM and 500 mM. Platelets (green: CD41), fibrinogen (red). (D) In bare and endothelialized channels, IL4-R/Iba blood forms fewer aggregates at 50 mM FeCl3 than WT blood, and the channel remains patent. Significance is denoted by an asterisk (*), and was measured by a 2-tailed, Student t test. Representative image of WT blood and IL4-R/Iba blood aggregation in the microfluidic channel. All scale bars represent 50 μm; N = 4.