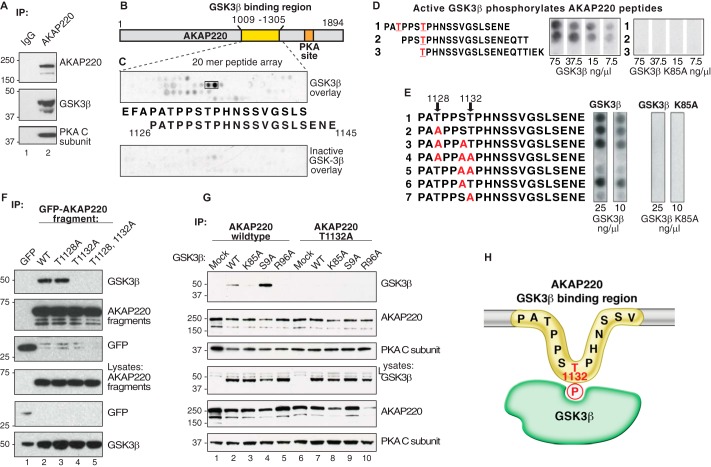
FIGURE 1.
The GSK3β phospho binding site on AKAP220 is Thr-1132. A, HEK293 cell lysates were immunoprecipitated (IP) with anti-AKAP220 antibody, and isolated complexes were analyzed by Western blot. B and C, peptide mapping of the GSK3β binding region, residues 1009–1305 of the mouse AKAP220 protein. Overlapping peptides (20-mers displaced by 3 amino acids sequentially) were synthesized onto a cellulose membrane and overlaid with purified GSK3β. D, Thr-1128 and Thr-1132 were predicted GSK3β phosphosites by Scansite analysis (red). Peptides containing these sites were synthesized by peptide array, and membranes were incubated with [γ32P]ATP and increasing concentrations of purified GSK3β or GSK3β K85A (kinase-dead). GSK3β phosphorylation was detected by autoradiography. E, AKAP220 1126–1145 peptides with candidate serine and threonine residues substituted with alanine were tested for GSK3β phosphorylation to locate the phosphosite. F, lysates from cells expressing either the GFP-AKAP220 1009–1305 fragment or one of the T1128A, T1132A, or the T1128A/T1132A mutant fragments were immunoprecipitated with anti-GFP antibody. Co-precipitation of endogenous GSK3β was assessed by immunoblot with an anti-GFP antibody. G, full-length V5-AKAP220 wild type or V5-AKAP220-T1132A were co-expressed in HEK293 cells with HA-GSK3β wild type, HA-GSK3β-K85A, HA-GSK3β-S9A, or HA-GSK3β-R96A. Immunocomplexes were isolated with a V5-antibody and probed for HA-GSK3β by Western blot. H, schematic representation of the AKAP220 Thr-1132 phosphosite binding to active GSK3β.