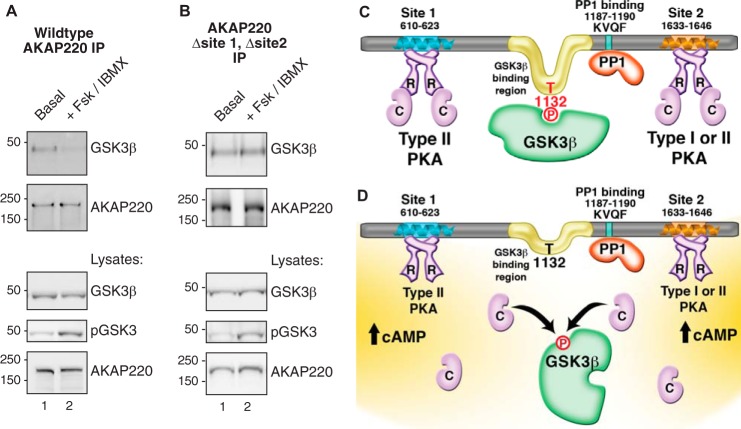
FIGURE 6.
GSK3β dissociates from AKAP220 complexes in response to cAMP. A, HEK293 cells were stimulated with 10 μm FSK and 75 μm IBMX for 10 min at 37 °C to increase intracellular cAMP and activate PKA. AKAP220 immunocomplexes were isolated from cell lysates and probed by immunoblot. GSK3β binding was decreased in stimulated cells. Control blots confirmed equal expression and pulldown of AKAP220. An antibody specific for phospho-GSK3β(Ser-9) detected a higher signal in the treated lysates, confirming stimulation. No phospho-GSK3β(Ser-9) was detected in the pulldown assays. IP, immunoprecipitation. B, the experiment was repeated with a mutant form of AKAP220 that does not bind PKA. Dissociation of GSK3β was blunted in these conditions. C, schematic representation of enzyme binding sites on AKAP220. PKA can bind to two sites. Site 1 is RII-selective, and site 2 has dual-specificity for RI or RII. One copy of active GSK3β interacts with AKAP220 between residues 1126 and 1145, a region containing the GSK3β phosphosite at Thr-1132 that is required for GSK3β binding. Additionally, PP1 binds to the KVQF motif at residues 1187–1190. D, proposed model of cAMP responsive GSK3β dissociation from AKAP220. In response to cAMP, anchored PKA is activated, and the C subunits are released. Anchored GSK3β is phosphorylated, preventing it from phosphorylating Thr-1132 causing its release from the complex. Anchored PP1 may relieve PKA-mediated inhibition of GSK3β by dephosphorylating Ser-9, providing bi-directional control of AKAP220 complex formation in response to cAMP.