FIGURE 9.
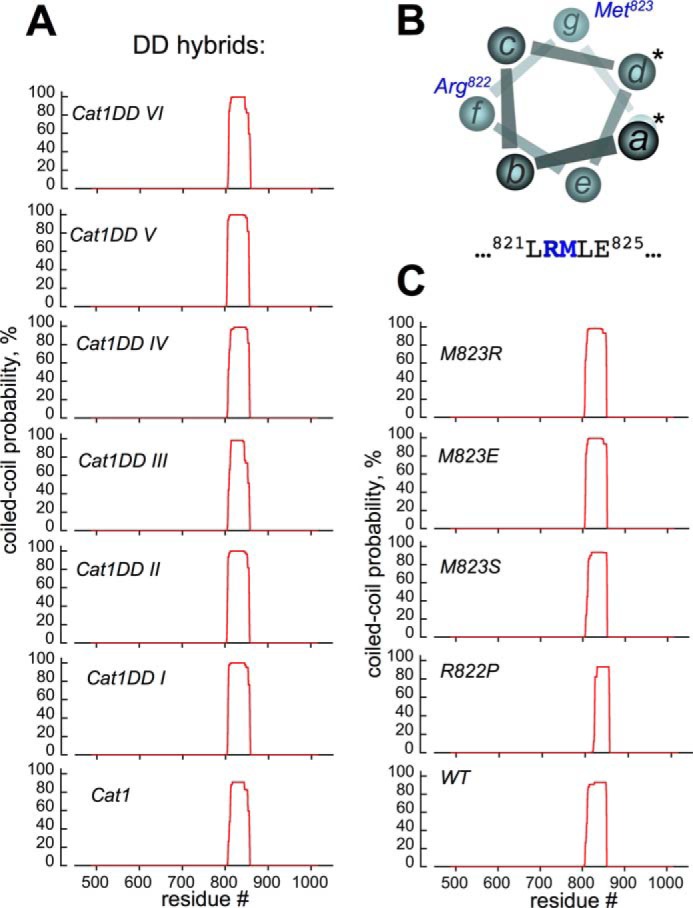
Probabilities of coiled coil dimerization remain high for RetGC1/NPRA hybrids and RetGC1 point mutants. The primary structure of the cyclase cytoplasmic domain was tested for the probability of forming a coiled coil dimer at different positions (red line) using conventional MARCOIL software utilizing the standard MTIDK matrix (45, 46); positions of the residues are numbered relative to the Met1 of the leader peptide encoded by the human GUCY2D gene. A, top to bottom, RetGC1/NPRA chimeras Cat1DD VI–I and RetGC1/NPRA chimera Cat1. B, in the first coiled coil heptad of the RetGC1, neither Arg822 nor Met823 (positions f and g, respectively) can make direct contact between two coils (positions a and d making the coiled coil contacts are marked with asterisks). The MARCOIL probability of Met823 or Arg822 to occupy the a or d position was below 0.1%. C, point mutations (top to bottom), M823R/E/S or R822P substitutions do not prevent coiled coil dimerization of the RetGC1 DD.
