FIGURE 8.
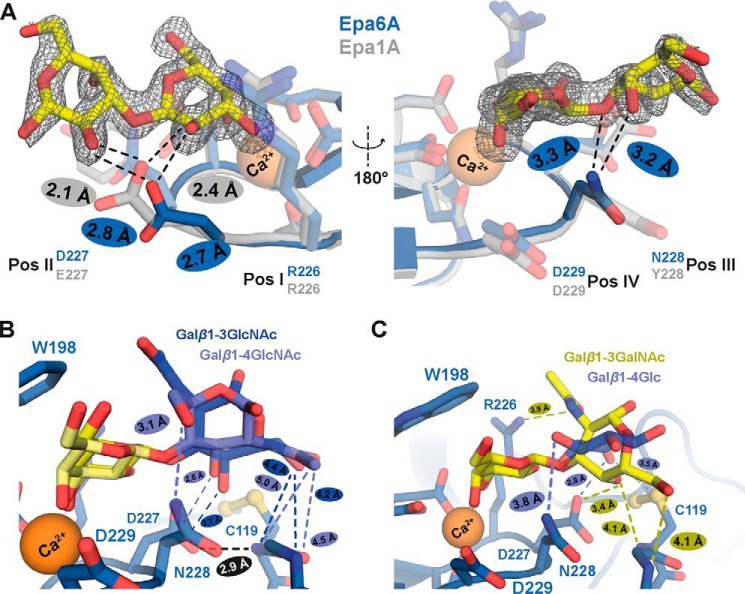
Role of CBL2 in conferring ligand discrimination by Epa1A and Epa6A. A, structural basis for α-/β-discrimination by Epa1A and Epa6A. Shown is a superposition of the structure of Epa1A (gray) with the structure of the Epa6A·α-galactobiose complex (blue). Higher selectivity of Epa6A toward α-linked galactosides is achieved by the sterically preferred Asp residue at CBL2 position II (Asp-227) in comparison with the sterically more demanding Glu residue at the corresponding position of Epa1A (Glu-227). In addition, a hydrogen bond is observed between α-galactobiose and the CBL2 position III (Asn-228) of Epa6A, which is absent in the Epa1A that carries a Tyr at this position (Tyr-228). B, ligand discrimination by Epa6A. Comparison of Galβ1–3GlcNAc (dark blue) and Galβ1–4GlcNAc (light blue) bound to Epa6A reveals divergent ligand-binding patterns. Whereas the backbone of residue Cys-119 of Epa6A interacts with the 6-OH group of the secondary sugar in the case of Galβ1–3GlcNAc, the same residue of Epa6A contacts the N-acetyl group of the GlcNAc moiety of Galβ1–4GlcNAc. C, ligand discrimination by Epa6A. Comparison of Galβ1–3GalNAc (yellow) with Galβ1–4Glc (light blue) bound to Epa6A reveals highly deviating binding patterns. The secondary carbohydrate is rotated by 90° and different amino acid residues of Epa6A are used for interaction.