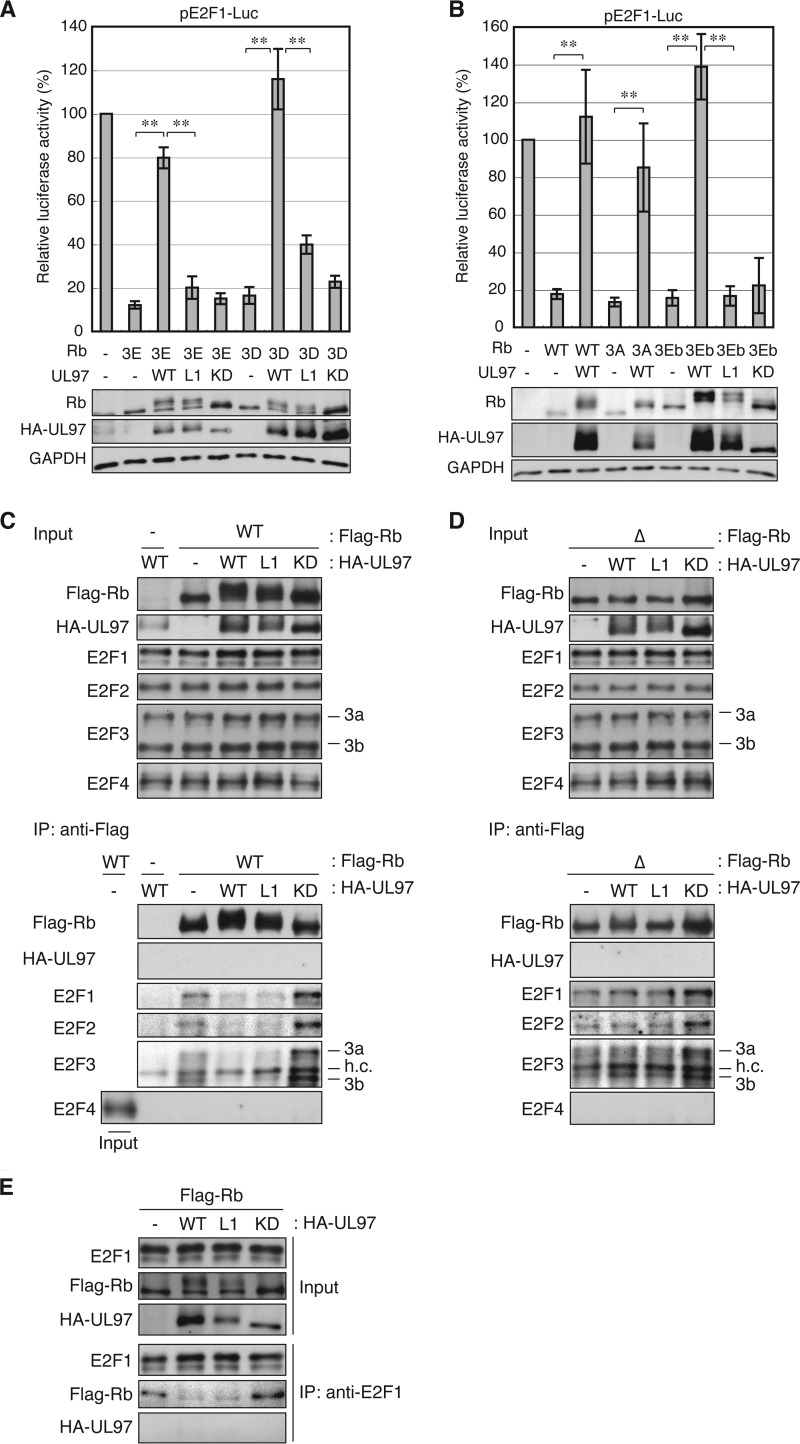
FIGURE 6.
Underphosphorylation of Rb by UL97-L1m neither mediates sustained repression of E2F-responsive promoters by Rb nor affects disruption of Rb-E2F complexes. A, Saos-2 cells were transfected with a luciferase reporter driven by the E2F1 promoter together with an empty vector (−) or an expression plasmid for Rb alleles with specific phosphomimetic residue substitutions (3E, T373E/T821E/T826E; 3D, T373D/T821D/T826D) and the indicated allele of UL97. Lysates harvested 48 h after transfection were analyzed for luciferase activity (top) and protein expression with the indicated antibodies (bottom). Luciferase activity was normalized to total protein concentration and is presented relative to the activity of the reporter without Rb or UL97 (set at 100%). Error bars denote the S.D. **, p < 0.01. B, luciferase and Western blot analyses were performed as in A except WT Rb or Rb alleles with different phosphomimetic (3Eb, T356E/T821E/T826E) or non-phosphorylatable (3A, T356A/T821A/T826A) residue substitutions were also included. C, Saos-2 cells were transfected with expression plasmids for wild-type FLAG-tagged Rb and the indicated allele of UL97. Lysates harvested 48 h after transfection were subjected to immunoprecipitation (IP) with the FLAG antibody. Input lysates and immunoprecipitates were analyzed by Western blotting with the indicated antibodies. h.c., heavy chain. D, immunoprecipitation experiment as in C except an Rb allele in which CDK consensus phosphorylation residues were replaced with alanines (Δ) was transfected. E, immunoprecipitation experiment as in C except the E2F1 antibody was used for immunoprecipitation. KD, kinase-deficient.