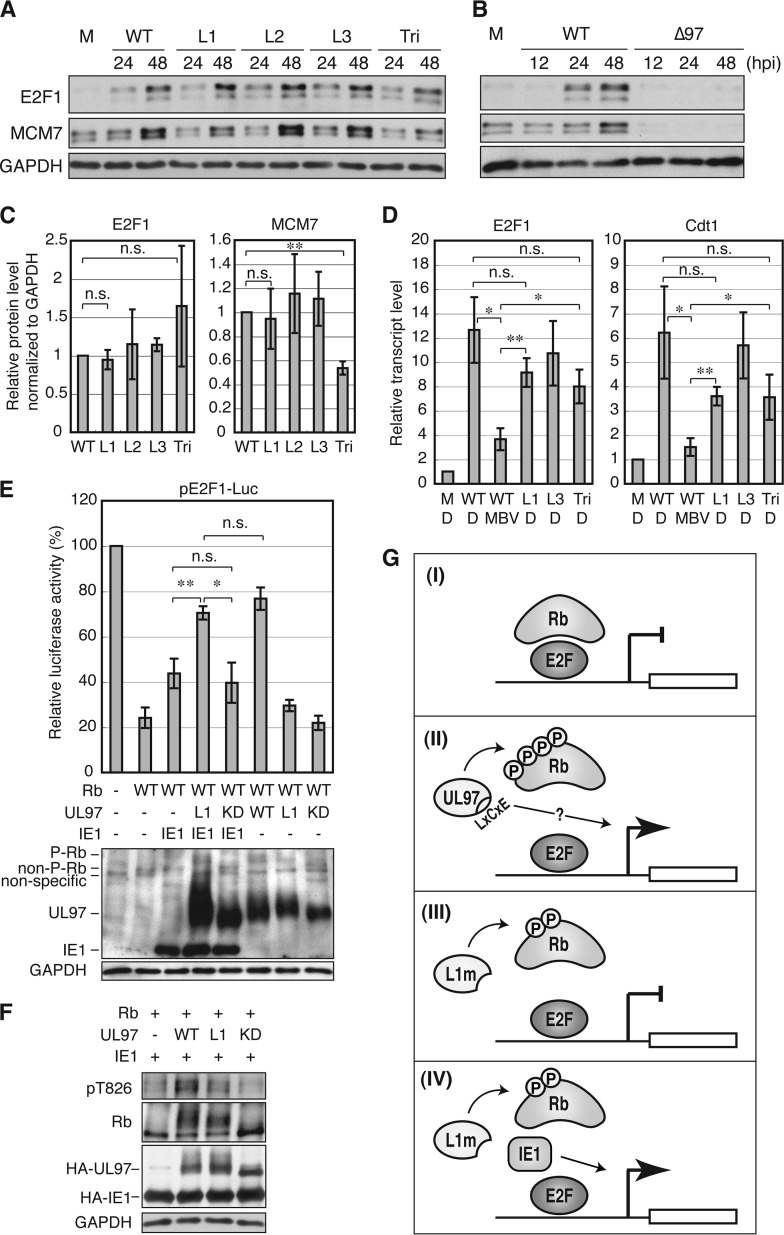
FIGURE 8.
The first UL97 LXCXE motif is dispensable for expression of the E2F-responsive genes E2F1, MCM7, and Cdt1 during HCMV infection likely because of complementation from the viral IE1 protein. A, serum-starved HFFs were mock-infected (M) or infected with WT HCMV or the indicated UL97 mutant virus at a multiplicity of infection of 1. At the indicated hour postinfection (hpi), protein lysates were harvested and analyzed by Western blotting with the indicated antibodies. B, Western blot analysis conducted as in A except during either WT or UL97-null (Δ97) HCMV infection. C, E2F1 and MCM7 protein levels at 48 h postinfection from the experiment shown in A were quantified and normalized to GAPDH protein, and values are presented relative to the value in wild-type HCMV-infected cells (set at 1). Error bars denote the S.D. **, p < 0.01; n.s., not significant. D, serum-starved HFFs were infected with each virus at a multiplicity of infection of 2 in the presence of maribavir (MBV) or DMSO (D). E2F1 and Cdt1 mRNA levels at 48 h postinfection were analyzed by quantitative PCR and normalized to GAPDH mRNA level. Values are presented relative to the value in mock infection (M) (set at 1). Error bars denote S.D. *, p < 0.05; **, p < 0.01; n.s., not significant. E, Saos-2 cells were transfected with a luciferase reporter driven by the E2F1 promoter together with an empty vector (−) or an expression plasmid for Rb, the indicated allele of UL97, or HCMV IE1. Lysates harvested 48 h after transfection were analyzed for luciferase activity (top) and protein expression with the indicated antibodies (bottom). Luciferase activity was normalized to total protein concentration and is presented relative to the activity of the reporter without Rb, UL97, or IE1 (set at 100%). P-Rb, hyperphosphorylated Rb; non-P-Rb, non-phosphorylated Rb. F, Saos-2 cells were transfected as in E. Lysates harvested 48 h after transfection were analyzed by Western blotting with the indicated antibodies. G, model for Rb inactivation by UL97. I, Rb represses E2F-responsive transcription. II, UL97 induces E2F-responsive transcription both by Rb phosphorylation-dependent disruption of Rb-E2F complexes, which is an LXCXE-independent event, and by an unknown L1 LXCXE-dependent function. III, a UL97 mutant with a non-functional L1 motif (UL97-L1m) induces partial phosphorylation of Rb and disruption of Rb-E2F complexes but fails to activate Rb-repressed E2F-responsive transcription. IV, the inability of UL97-L1m to activate Rb-repressed E2F-responsive transcription is complemented by HCMV IE1 through an unknown mechanism. KD, kinase-deficient.