FIGURE 9.
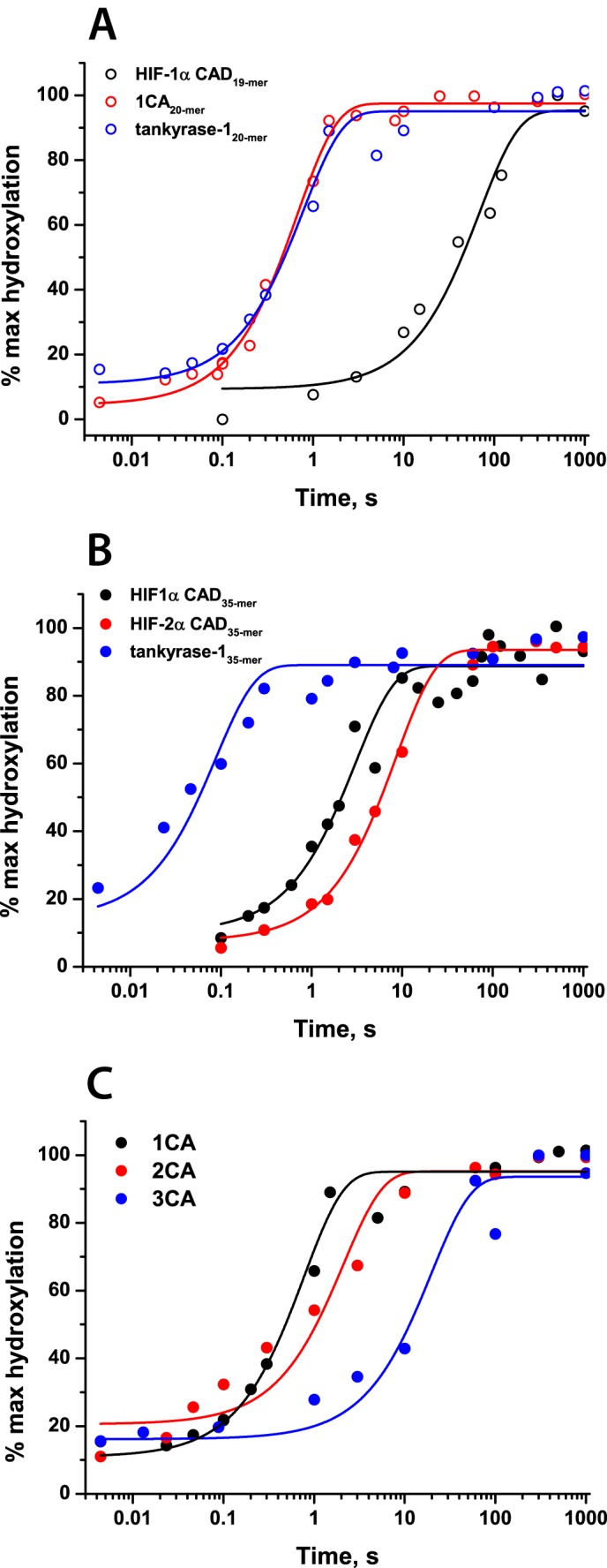
Rapid quench-flow experiments showing FIH-catalyzed hydroxylation of HIF-1/2α CAD and ARD substrates. Reaction mixtures containing anaerobic 0.5 mm apo-FIH, 0.4 mm Fe(II), 5 mm 2OG, 1 mm peptide in HEPES 50 mm (pH 7.5) were rapidly mixed with O2-saturated buffer at 5 °C in a 1:1 ratio and then quenched with 1% CF3CO2H at defined time points. The data were fitted with the function, y = a·(1 − exp(−bx)) + c. Hydroxylation levels were assessed by MALDI-TOF-MS. A, hydroxylation of HIF-1α CAD, 1CA, and tankyrase-1 peptide substrates (19- and 20-mers); B, hydroxylation of HIF-1α, HIF-2α, and tankyrase-1 35-mer peptide substrates; C, hydroxylation of 1CA, 2CA, and 3CA. Data plotted were cumulatively acquired over the course of 1–3 separate experiments.
