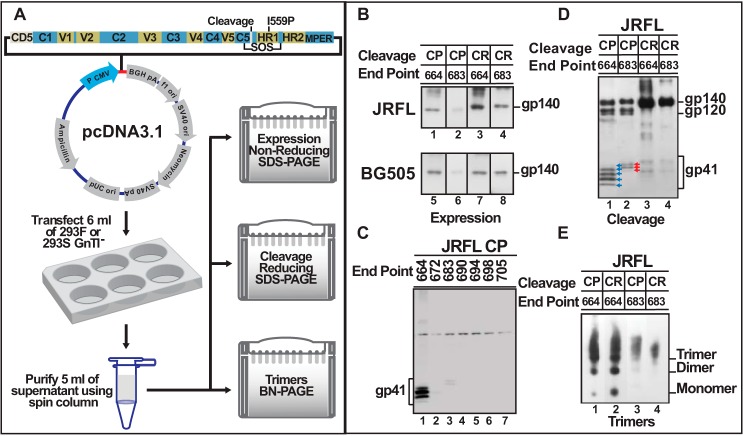
FIGURE 3.
Truncations beyond aa 664 produce few or no gp140 trimers. A, a screening strategy to optimize recombinant gp140 production. B, D, and E, the secreted gp140 was captured by adding Strep-Tactin beads to the culture medium and eluted with 2.5 mm desthiobiotin. The same amount of the eluted sample was used to determine the efficiency of expression (B), cleavage and gp41 glycosylation (D), and trimer production (E). C, the efficiency of expression was further assessed by directly using the supernatant without the addition of Strep-Tactin beads. The samples were electrophoresed under reducing conditions so that gp41 would be cleanly separated and fully available for interaction with the Strep-tag mAb used for Western blotting. B, non-reducing SDS gel comparing the production of uncleaved and cleaved gp140 in the culture medium for aa truncations 664 and 683. C, reducing SDS gel comparing the production of gp140 recombinants truncated at various aa positions at the C terminus. End point numbers correspond to the aa at the end of the C terminus. The same volume of the supernatant was loaded in each lane. Note the presence of a nonspecific band in similar amounts in all of the lanes at about one-third of the distance from the top of the gel. D, reducing SDS gel of uncleaved and cleaved gp140 proteins truncated at aa 664 and 683. Blue and red arrows, differentially glycosylated gp41 ectodomain bands of gp140 proteins truncated at amino acids 664 and 683, respectively. E, BN gel of Strep-Tactin-purified gp140 samples. B, D, and E, Western blots using mouse anti-gp140 polyclonal antibody. C, Western blot using Strep-tag II-specific mAb. Data shown are for GnTI−-produced JRFL gp140. Similar patterns were observed for 293F-produced JRFL gp140 and for BG505 gp140 produced in GnTI− or 293F cells.