FIGURE 3.
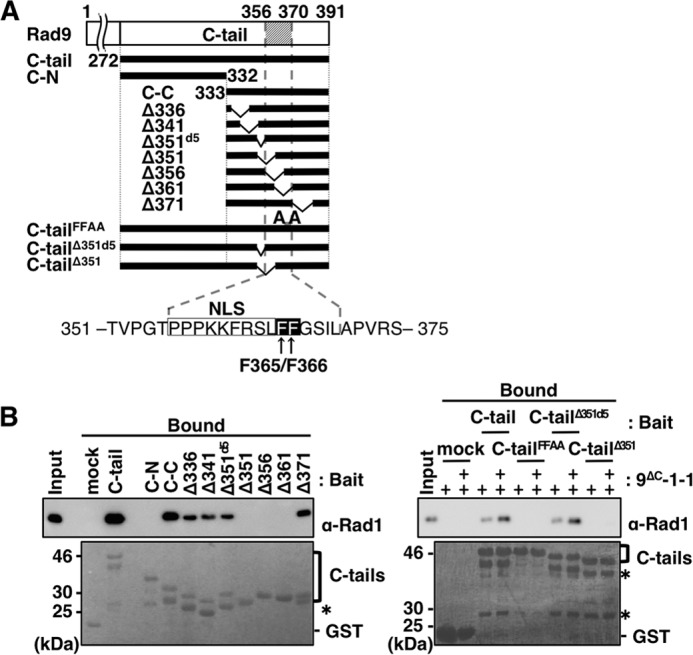
Analyses of amino acid sequence of C-tail necessary for its binding to 9ΔC-1-1. A, schematic illustration of human C-tail and its deletions used in this study. C-tail, C-N, and C-C contain aa 272–391, aa 272–332, and aa 333–391, respectively. Constructs Δ336, Δ341, Δ351d5, Δ351, Δ356, Δ361, and Δ371 harbor internal deletions of aa 336–345, aa 341–350, aa 351–355, aa 351–360, aa 356–365, aa 361–370, and aa 371–380 from C-C, respectively; C-tailΔ351d5 and C-tailΔ351 are the same deletions as Δ351d5 and Δ351, respectively, from the C-tail. C-tailFFAA harbors Ala substitutions at both Phe-365 and Phe-366, which were expressed as fusions with GST/FLAG tags at their N-terminal portion. Below is the sequence of the C-tail, including the 15-aa stretch (aa 351–375) required for interaction with CRS. The NLS and FF motif (Phe-365/Phe-366) are boxed in white and black, respectively. B, glutathione-Sepharose beads pre-bound with GST only (mock), GST/FLAG-tagged C-tail (C-tail), C-tail mutant (C-tailFFAA), or its deletions (C-N, C-C, Δ336, Δ341, Δ351d5, Δ351, Δ356, Δ361, Δ371, C-tailΔ351d5, and C-tailΔ351) were mixed with 10 pmol alone (left panel) or 5 and 10 pmol (+, and ++, respectively) (right panel) of purified 9ΔC-1-1, and incubated at 4 °C for 1 h. After washing the beads, 50 fmol (left panel) and 100 fmol (right panel) of purified 9ΔC-1-1 and 10% (left panel) or 20% (right panel) of the bound fractions were analyzed by immunoblotting using anti-Rad1 antibody (upper panels) and staining with Ponceau S for the C-tail and its deletions (lower panels). Asterisks indicate degraded C-tail fragments.
