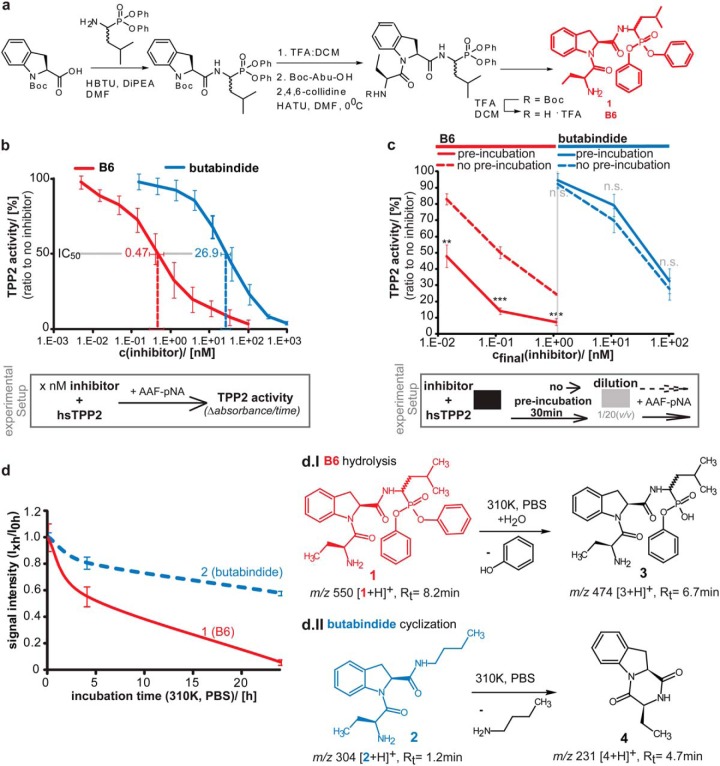
Fig. 1.
B6—A new irreversible TPP2 inhibitor- synthesis, structure, IC50 value, irreversibility, and stability. (A) Schematic synthesis strategy of B6. Detailed description of B6 synthesis and intermediate products can be found in the supplementary experimental procedure. Structure of B6 (compound 1) is marked in red. (B) IC50 value of B6 for inhibiting human TPP2 (hTPP2) in vitro. Different concentrations of B6 (red) and in parallel butabindide (blue) were incubated in vitro with purified hTPP2. The activity was determined via the TPP2 substrate AAF-pNA and measured as time-dependent fluorescence of free pNA (a.u./min). An average line is shown. A schematic experimental setup is presented below the graph. Activity relative to noninhibited TPP2 in percentage is plotted against inhibitor concentration (n = 3, ±S.D.). IC50, as determined by the tangent equation of the regression function for B6 (red) and as reference butabindide (blue) are indicated. (C) Binding of B6 to TPP2 is irreversible compared with butabindide. Different concentrations of B6 (red curves, left side) and in parallel butabindide (blue curve, right side) were applied to purified human TPP2 and either preincubated (30 min, dashed line) and then diluted 1/20 (v/v) or directly diluted (solid curve). The activity was determined via the TPP2 substrate AAF-pNA and measured as time-dependent fluorescence of free pNA (a.u./min). A schematic experimental setup is presented below the graph. Activity relative to noninhibited TPP2 in percentage (n = 3, ±S.D.) is plotted against final inhibitor concentration after dilution. (D) Stability of B6 under mimicked physiological conditions (310 K, in PBS). Stock solutions of B6 or butabindide (10 mm in DMSO) were diluted in PBS, pH 7.2 (cfinal = 20 μm), an aliquot (200 pmol) was directly(0 h), after 4 h, and after 24 h incubation at 310 K applied to LC-MS. Graph shows signal intensity (MS) of B6 ([1+H]+ m/z 550) and butabindide ([3+H]+ m/z 304) normalized to 0 h value (n = 3, ±S.D.). d.I and d.II represent the chemical reactions of B6 and butabindide as observed under mimicked physiological conditions and determined by identification of the products using mass spectrometry.