Figure 7.
CDC42 Inhibition Impairs Retinal Vessel Sprouting and Branching Similarly to ABL Inhibition but Differently to Loss of NRP1-Dependent VEGF-A Signaling
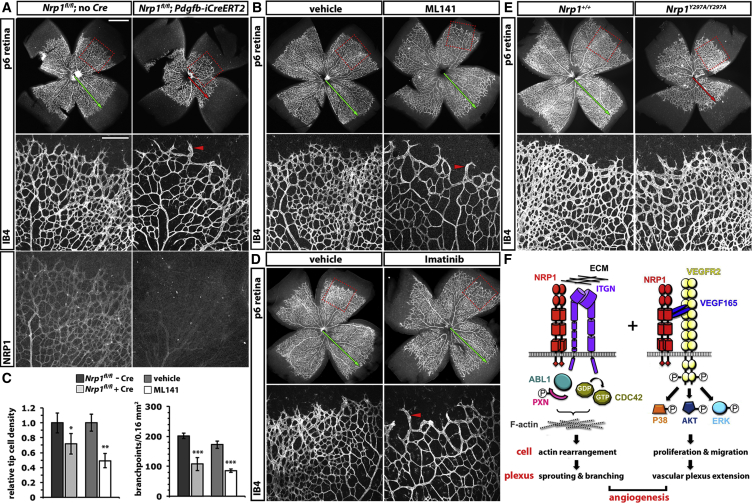
(A–C) Comparison of retinal vascular defects after endothelial NRP1 loss or CDC42 inhibition. IB4 labeling of P6 retinal vasculature from littermate Nrp1fl/fl mice lacking Cre (n = 8) or expressing Pdgfb-iCre-ERT2-Egfp (n = 6) after daily tamoxifen injection from P2 to P5 (A) or from littermate wild-type mice treated daily from P2 to P5 with vehicle (n = 3) or ML141 (n = 4) (B). In (A), retinas were co-immunolabeled for NRP1 to demonstrate knockdown in mutants. (C) Quantification of filopodial bursts at the vascular front and branchpoints behind the vascular front; mean ± SD; asterisks indicate ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
(D and E) Comparison of retinal vascular defects after ABL kinase inhibition or loss of VEGF-A binding to NRP1. IB4 labeling of P6 retinal vasculature from littermate wild-type mice treated daily from P2 to P5 with vehicle or Imatinib (D) or Nrp1Y297A/Y297A mice lacking VEGF-A binding to NRP1 and wild-type littermates (E).
For (A), (B), (D), and (E), the top panels show low-magnification images of retinal flat mounts (scale bar, 1 mm) and boxed areas are shown at higher magnification below each panel (scale bar, 200 μm). The green arrow indicates normal vascular extension, the red arrows defective vascular extension, and the arrowheads abnormally long and wide sprouts without lateral protrusions or connections.
(F) Schematic representation of NRP1 roles in angiogenesis. NRP1 enables the ECM-dependent activation of ABL1 and CDC42 in addition to its classical role as a VEGFR2 co-receptor in VEGF-A signaling.