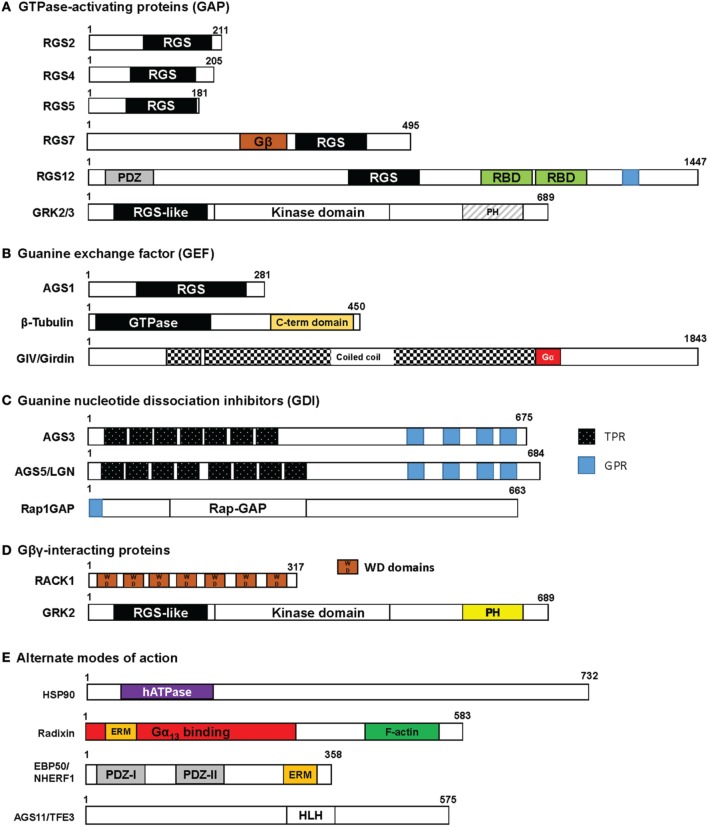
Figure 2.
Protein domain structure in accessory proteins. Each of the accessory proteins described in this review are drawn with their respective domains using the wild-type protein sizes obtained from a consensus human sequence. The protein structures are categorized by their putative biological roles in regulating G-protein function as shown in Figure 1: (A) GAP; (B) GEF; (C) GDI; and (D) Gβγ interaction. G-protein subunits have been determined to interact within the RGS box, Gβ, GPR, PH, or WD domains. In some accessory proteins listed in (E) “Alternate mode of action,” the radixin protein has been identified to bind in the N-terminal part of the protein and EBP50 interacts with Gαq in the PDZ domains. The binding sites for HSP90 and AGS11 have yet to be determined. RGS, Regulator of G-protein Signaling box; RBD, Ras binding domain; TPR, tetratricopeptide repeat; GPR, G-protein regulatory motif; WD, WD40 repeat; PDZ, PSD-95/Drosophila disk large/ZO-1 domains; PH, pleckstrin homology domain; hATPase, human ATPase; ERM, ezrin-radixin-moesin binding domain; HLH, helix-loop-helix.