Abstract
Background
Enhancement of myocardial glucose uptake may reduce fatty acid oxidation and improve tolerance to ischemia. Hyperglycemia, in association with hyperinsulinemia, stimulates this metabolic change but may have deleterious effects on left ventricular (LV) function. The incretin hormone, glucagon-like peptide-1 (GLP-1), also has favorable cardiovascular effects, and has emerged as an alternative method of altering myocardial substrate utilization. In patients with coronary artery disease (CAD), we investigated: (1) the effect of a hyperinsulinemic hyperglycemic clamp (HHC) on myocardial performance during dobutamine stress echocardiography (DSE), and (2) whether an infusion of GLP-1(7-36) at the time of HHC protects against ischemic LV dysfunction during DSE in patients with type 2 diabetes mellitus (T2DM).
Methods
In study 1, twelve patients underwent two DSEs with tissue Doppler imaging (TDI)—one during the steady-state phase of a HHC. In study 2, ten patients with T2DM underwent two DSEs with TDI during the steady-state phase of a HHC. GLP-1(7-36) was infused intravenously at 1.2 pmol/kg/min during one of the scans. In both studies, global LV function was assessed by ejection fraction and mitral annular systolic velocity, and regional wall LV function was assessed using peak systolic velocity, strain and strain rate from 12 paired non-apical segments.
Results
In study 1, the HHC (compared with control) increased glucose (13.0 ± 1.9 versus 4.8 ± 0.5 mmol/l, p < 0.0001) and insulin (1,212 ± 514 versus 114 ± 47 pmol/l, p = 0.01) concentrations, and reduced FFA levels (249 ± 175 versus 1,001 ± 333 μmol/l, p < 0.0001), but had no net effect on either global or regional LV function. In study 2, GLP-1 enhanced both global (ejection fraction, 77.5 ± 5.0 versus 71.3 ± 4.3%, p = 0.004) and regional (peak systolic strain −18.1 ± 6.6 versus −15.5 ± 5.4%, p < 0.0001) myocardial performance at peak stress and at 30 min recovery. These effects were predominantly driven by a reduction in contractile dysfunction in regions subject to demand ischemia.
Conclusions
In patients with CAD, hyperinsulinemic hyperglycemia has a neutral effect on LV function during DSE. However, GLP-1 at the time of hyperglycemia improves myocardial tolerance to demand ischemia in patients with T2DM.
Trial Registration: http://www.isrctn.org. Unique identifier ISRCTN69686930
Keywords: Hyperglycemia, Coronary disease, Diabetes mellitus, Glucagon-like peptide, Stress echocardiography
Background
Hyperglycemia is common in patients presenting with acute coronary syndromes (ACS) and a powerful predictor of morbidity and mortality [1]. Although it has a number of direct detrimental effects on the ischemic myocardium [2], hyperglycemia is also representative of an acute metabolic stress characterised by high adrenergic-mediated levels of free fatty acids (FFA), insulin resistance and impaired glucose utilization [3]. Attempts to modulate these metabolic derangements with insulin-based strategies have failed to demonstrate consistent clinical benefits [4, 5] and are yet to be established in the management of patients with ACS [6, 7]. There is therefore an ongoing requirement for the development of new pharmacological therapies to address this problem.
Glucagon-like peptide-1 (GLP-1) is an incretin hormone which regulates glucose metabolism by stimulating insulin secretion, suppressing glucagon and promoting glucose uptake into muscle and adipose tissue. These effects are dependent on the prevailing glucose concentration so that the risk of hypoglycemia is minimized [8]. As a result, the need for intensive monitoring and concomitant glucose infusions associated with insulin-based metabolic strategies is obviated [9]. As well as these glucoregulatory effects, GLP-1 also has binding sites in the heart, and clinical studies demonstrating the cardioprotective properties of incretin-modulating agents are continuing to emerge [10–16]. GLP-1 therefore appears attractive as a potential therapeutic adjunct for metabolic manipulation in patients with ischaemic heart disease (IHD).
We have previously used a model of hyperinsulinemic euglycemic clamping (HEC) during dobutamine stress echocardiography (DSE) to demonstrate that alteration of the myocardial metabolic environment in patients with obstructive coronary artery disease (CAD) can attenuate left ventricular (LV) ischemic dysfunction, especially in those with insulin resistance [17]. However, the effects of hyperinsulinemic hyperglycemia (HH) in this setting have not been investigated and remain unclear. This study was therefore undertaken to answer two questions relating to patients with CAD. Firstly, we sought to investigate the effects of HH on LV performance during dobutamine stress. Secondly, we sought to investigate the effect of GLP-1 modulation on myocardial function in the setting of hyperglycemia in patients with type 2 diabetes mellitus (T2DM).
Methods
Study population
Patients with obstructive CAD (at least one proximal stenosis >70% in at least one epicardial coronary artery) and preserved resting LV systolic function were invited to participate. All patients had undergone recent coronary angiography before enrolment in the study. Exclusion criteria included LV ejection fraction (EF) <40% (either on echocardiography or LV angiography), regional wall motion abnormalities at rest, a history of previous myocardial infarction within the preceding 3 months, conduction abnormalities, valvular heart disease, patients taking insulin, dipeptidyl peptidase-4 (DPP4) inhibitors or GLP-1 receptor agonists, and those with permanent pacemakers. The study protocol was approved by the local ethics committee (REC number 08/H0304/68), and conformed with the guidelines set out in the Declaration of Helsinki. All participants gave written informed consent. The trial number was ISRCTN69686930
Study design
Two separate studies were undertaken:
Study 1: effects of hyperglycemia on myocardial performance during dobutamine stress
Each subject underwent two DSEs, performed approximately one week apart. One DSE, determined randomly, was performed during the steady-state phase of a hyperinsulinemic hyperglycemic clamp (HHC) and the other DSE acted as a control (Fig. 1).
Fig. 1.
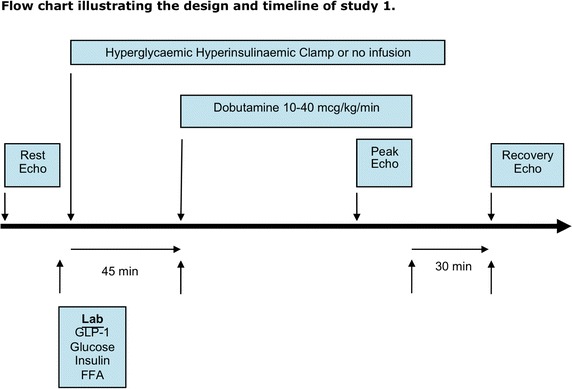
Flow chart illustrating the study design and timeline of study 1.
Study 2: effects of GLP-1 (7-36) on myocardial performance during dobutamine stress in the setting of hyperglycemia
Consecutive patients with T2DM underwent DSE during the steady-state phase of a HHC on two separate occasions, approximately one week apart. One DSE, determined randomly, was performed during an intravenous infusion of GLP-1 (7-36) amide (Bachem, Germany) at a dose of 1.2 pmol/kg/min, and the other DSE (control HHC) during an intravenous infusion of normal saline (Fig. 2).
Fig. 2.
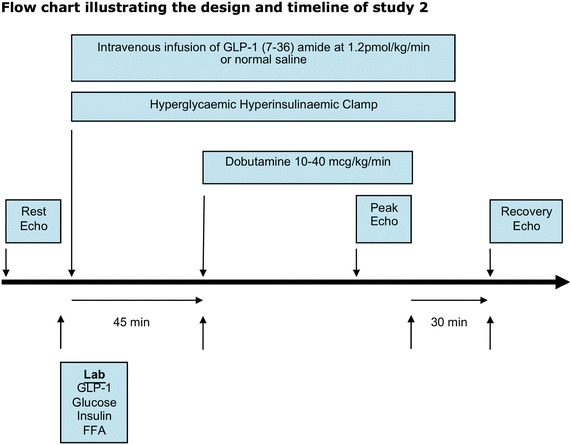
Flow chart illustrating the study design and timeline of study 2.
Hyperinsulinemic, hyperglycemic clamp
The HHCs were performed as described previously [18]. After patients had fasted overnight, a cannula was inserted into a vein in the antecubital fossa for infusion of glucose. A second cannula was inserted in the opposite arm, which was arterialized using a heating pad set at 50°C. In order to raise the blood glucose concentration to approximately 13 mmol/L, a bolus injection of 0.3 mg/kg of 25% glucose was given over the first minute of the clamp. Once the bolus was administered, a maintenance infusion of 20% glucose was commenced at 5 mg/kg/min (study 1) or 2.5 mg/kg/min (study 2) and this rate was kept constant for the first 10 min. Blood glucose levels were sampled every 5 min from the opposite cannula and analyzed using the glucose oxidase method (YSI 2300, YSI Life Sciences, Ohio, USA). Thereafter, the glucose infusion rate was adjusted according to the plasma glucose concentration, aiming to maintain it at 13 mmol/L. The clamp was then continued for a minimum of 45 min. Once steady state was achieved, as indicated by three consecutive blood glucose values that were within 5% of one another, the subjects underwent DSE as described below. After cessation of dobutamine, the glucose infusion was continued for 15 min and then gradually reduced over the next 30 min to avoid rebound hypoglycemia.
Dobutamine stress echocardiography
A standard clinical protocol for DSE was used. Subjects attended for both studies after an overnight fast and their beta-blocker was withheld for 48 h prior to their attendance. Oral hypoglycemic agents were omitted on the morning of the study. Dobutamine was administered intravenously via the antecubital fossa using an infusion pump in incremental doses (10 µg/kg/min initially, then increased at 3-min intervals to 20, 30 and 40 µg/kg/min if tolerated) and if necessary, up to 2 mg of atropine was given to achieve the target heart rate. Subjects underwent continuous 12-lead ECG monitoring, with blood pressure being recorded at baseline and at the end of each stage of dobutamine. Criteria for stopping the test were achievement of target heart rate of (220-age) × 0.85 bpm, ischemic ECG changes (>2 mm ST depression), angina, systolic blood pressure increase to >240 mmHg or decrease to <100 mmHg, and severe arrhythmias. Two-dimensional echocardiography (Vivid 7, GE Medical Systems) was performed with the patient in the left recumbent position, and images were recorded at rest, peak stress and in recovery. Three cardiac cycles of the apical 4-, 3-, and 2-chamber views were captured with tissue Doppler imaging (TDI). The image sector width was kept as narrow as possible to maximize the frame rate. All recordings were made in gently held mid-expiration to minimize beat-to-beat variability, and the data were stored for subsequent off-line analysis (EchoPac Version 11, GE Medical Systems).
In addition to blood glucose sampling, blood samples were taken in the fasted state to measure insulin, FFA, and GLP-1(7-36) concentrations at baseline, at the steady state stage of the HHC immediately prior to commencement of the DSE (pre-DSE), during peak dobutamine stress and in the recovery phase at 30 min after cessation of dobutamine. The syringes for the collection of GLP-1 samples were pre-prepared with DPP-4 inhibitor (Millipore) to prevent GLP-1 degradation. Plasma GLP-1 levels were measured using a commercially available assay (Meso Scale Discovery, Rockville, MD, USA).
Echocardiographic analysis
The scans were analyzed off-line by a reviewer who was blinded to the treatment strategy. Regional wall LV motion was assessed using a 12-segment model comprising the base and mid level of six regional walls (anterior, anterolateral, anteroseptal, inferior, inferolateral, and inferoseptal) obtained from the three apical views. LV volumes and EF were calculated using the Simpson biplane method according to the guidelines of the American Society of Echocardiography. Global LV function was also assessed by mitral annular systolic velocity (MASV) averaged from six sites [19]. Peak systolic tissue velocity, and strain and strain rates were calculated from tissue Doppler velocity data averaged over three consecutive beats (Fig. 3). The timings of aortic valve opening and closure were made from the tissue Doppler waveform. The MYDISE study demonstrated that CAD could be diagnosed accurately and objectively from off-line measurements of myocardial velocities recorded by tissue Doppler echocardiography during dobutamine stress [20]. In particular, strain rate imaging has been shown to provide objective evidence of inducible ischemia [21], and may be a superior parameter to peak tissue velocity [22].
Fig. 3.
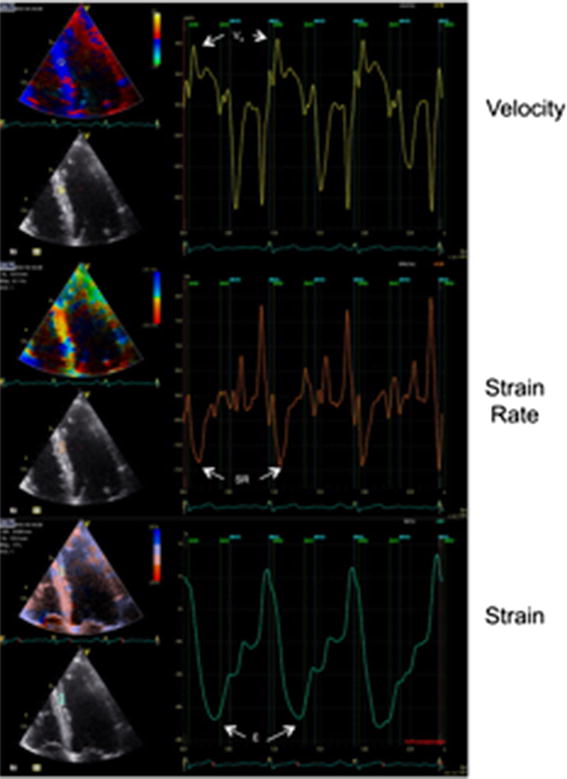
Myocardial velocity profile sampled from the mid inferoseptum with corresponding strain rate and strain curves.
A diameter stenosis of >70% stenosis on coronary angiography was considered hemodynamically significant. Myocardial segments were assigned to the perfusion territories of stenosed vessels, considering the left anterior descending coronary artery to supply the anterior and anteroseptal segments, the right coronary artery (when dominant) to supply the inferior and inferoseptal segments, and the circumflex artery to supply the anterolateral and inferolateral segments.
Statistics
The number of subjects had been calculated on the basis of previous work in our laboratory in patients with CAD, where EF increased from 70.8 ± 5.0% to 77.0 ± 4.4% when dobutamine stress was performed during an intravenous infusion of GLP-1 (7-36). To detect a change in global LVEF of 5% after dobutamine stress (standardized effect size of 1), 12 patients were required (paired t test, α = 0.05, β = 0.10). Interim analysis was conducted after the first seven participants to make adjustments to the sample size if required and therefore avoid patients undergoing DSE unnecessarily. Comparisons were made between HHC and control scans (study 1), or GLP-1 HHC and control HHC scans (study 2), with each patient acting as their own control. Continuous and discrete variables are expressed as mean ± standard deviation (SD) and compared by use of the paired Student’s t test (and repeated measures one-way ANOVA for regional function parameters) or the Wilcoxon signed-rank test where appropriate after testing for normality of distribution using the Shapiro–Wilk test. Categorical data are expressed as numbers (percentages) and compared by use of McNemar’s test. Two-tailed tests were used on all occasions, and a probability value of <0.05 was considered statistically significant. Intra- and inter-observer variations were calculated using the Bland–Altman method and expressed as the coefficient of variation (SD divided by the average value of the variable) ± the 95% limits of agreement.
Results
Study 1: effects of hyperglycemia on myocardial performance during dobutamine stress
Study population
Twelve patients were assigned to and completed study 1. The clinical characteristics of the subjects are shown in Table 1.
Table 1.
Clinical data of participants
| Study 1 (n = 12) | Study 2 (n = 10) | |
|---|---|---|
| Clinical characteristics | ||
| Age (year) | 64.8 ± 7.0 | 66.1 ± 4.6 |
| Male sex | 11 (92) | 10 (100) |
| Type 2 diabetes | 0 | 10 (100) |
| Weight (kg) | 84 (10) | 93 (14) |
| BSA (m2) | 2.0 ± 0.1 | 2.2 ± 0.2 |
| HOMA IR | 1.3 ± 0.8 | 2.7 ± 1.3 |
| HbA1c, (%) | – | 7.0 ± 0.7 |
| Coronary disease | ||
| LAD stenosis | 8 (67) | 6 (60) |
| LCx stenosis | 4 (33) | 2 (20) |
| RCA stenosis | 4 (33) | 7 (70) |
| Single vessel CAD | 8 (67) | 7 (70) |
| Two vessel CAD | 4 (33) | 1 (10) |
| Three vessel CAD | 0 | 2 (20) |
| Anti-anginal medications | ||
| B-blocker | 11 (92) | 6 (60) |
| Calcium channel antagonist | 1 (8) | 7 (70) |
| Long-acting nitrate | 8 (67) | 3 (30) |
| Nicorandil | 0 | 4 (40) |
| Oral hypoglycemic agents | ||
| Metformin | – | 4 (40) |
| Sulfonylurea | – | 4 (40) |
| Thiazolidinedione | – | 1 (10) |
Data are presented as mean ± SD or n (%). BSA body surface area, HOMA IR homeostasis model assessment of insulin resistance, LAD left anterior descending artery, LCx left circumflex artery, RCA right coronary artery, CAD coronary artery disease.
Dobutamine stress echocardiography
The two DSE were conducted 8.5 ± 3.9 days apart. There were no differences in the rate-pressure products at baseline, peak stress or recovery between the two scans (Table 2). Target heart rate was achieved in 6 (50%) subjects in the control study and 7 (59%) in the HHC study. As per the protocol, the dobutamine infusions in the remaining subjects were terminated early due to the development of angina.
Table 2.
Hemodynamic data during DSE scans in study 1
| Peak stress | Recovery | |||||
|---|---|---|---|---|---|---|
| Control | HHC | P | Control | HHC | P | |
| Heart rate (bpm) | 129 ± 15 | 128 ± 17 | 0.87 | 81 ± 15 | 78 ± 11 | 0.32 |
| Systolic blood pressure (mm Hg) | 153 ± 29 | 156 ± 22 | 0.79 | 136 ± 27 | 137 ± 19 | 0.86 |
| Diastolic blood pressure (mm Hg) | 76 ± 13 | 78 ± 11 | 0.86 | 81 ± 11 | 82 ± 11 | 0.90 |
| Rate-pressure product (mm Hg bpm) | 19,540 ± 3,719 | 19,900 ± 3,948 | 0.95 | 11,010 ± 2,977 | 10,730 ± 2,209 | 0.68 |
Biochemistry
At baseline, there were no differences in the plasma concentrations of glucose (4.8 ± 0.5 [HHC] versus 4.9 ± 0.6 mmol/l [control], p = 0.55), insulin (55 ± 39 [HHC] versus 86 ± 76 pmol/l [control], p = 0.18) or FFA (380 ± 320 [HHC] versus 429 ± 226umol/L [control], p = 0.36) between the HHC and control studies (Fig. 4). As intended, glucose concentrations at the steady state stage of the clamp (pre-DSE) were significantly higher than at baseline in the control study (13.3 ± 1.7 [HHC] versus 4.9 ± 0.6 mmol/l [control], p < 0.0001). This was associated with elevated insulin (378 ± 174 [HHC] versus 86 ± 76 pmol/l [control], p = 0.0007) and reduced FFA concentrations (77 ± 74 [HHC] versus 429 ± 226umol/l [control], p = 0.0003).
Fig. 4.
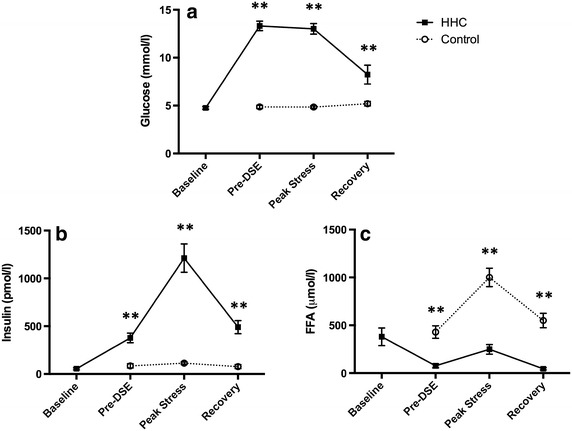
a–c Biochemical data (mean ± SEM) during the DSE scans in study 1 at baseline, pre-DSE, peak stress, and 30-min recovery. **p < 0.05.
At peak stress, the metabolic differences between the two visits persisted, with higher glucose (13.0 ± 1.9 [HHC] versus 4.8 ± 0.5 mmol/l [control], p < 0.0001) and insulin (1,212 ± 514 [HHC] versus 114 ± 47 pmol/l [control], p = 0.01) concentrations, and reduced FFA levels (249 ± 175 [HHC] versus 1,001 ± 333 μmol/l [control], p < 0.0001), in the clamp study compared with control. In the recovery phase at 30 min after cessation of dobutamine (15 min after cessation of the glucose infusion), glucose (8.2 ± 3.4 [HHC] versus 5.2 ± 0.8 mmol/l [control], p = 0.005) and insulin (491 ± 240 [HHC] versus 79 ± 72 pmol/l [control], p = 0.02) concentrations remained higher and FFA concentrations lower (46 ± 40 [HHC] versus 550 ± 262 μmol/l [control], p < 0.0001) in the HHC study, although the magnitude of the differences was not as great.
Global LV function
There were no differences in EF between the clamped and control studies at any stage (pre-DSE 66.3 ± 4.0 [HHC] versus 66.0 ± 4.7% [control], p = 0.76; peak stress 67.4 ± 11.0 [HHC] versus 69.1 ± 8.0% [control], p = 0.32; recovery 62.2 ± 4.0 [HHC] versus 63.5 ± 5.9% [control], p = 0.40). Assessment of global LV function by MASV confirmed these findings. The HHC was not observed to have any net effect on this parameter before, during or after dobutamine stress (pre-DSE 6.2 ± 1.4 [HHC] versus 5.8 ± 1.4 cm/s [control], p = 0.14; peak stress 10.4 ± 2.9 [HHC] versus 10.3 ± 2.9 cm/s [control], p = 0.68; recovery 6.2 ± 1.4 [HHC] versus 5.8 ± 1.4 cm/s [control], p = 0.12) (Fig. 5a).
Fig. 5.
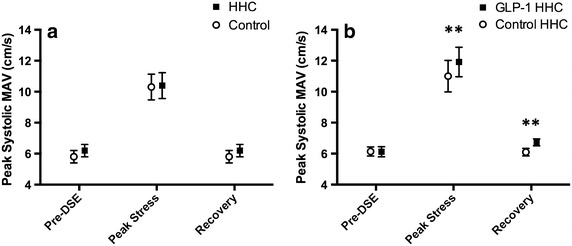
Global LV function assessed by peak systolic mitral annular velocity (mean ± SEM) at baseline, peak stress and 30-minute recovery in study 1 (a) and study 2 (b). **p < 0.05.
Regional wall LV function
For the 12 paired non-apical segments, there were no differences in peak systolic tissue velocity, peak systolic strain or peak systolic strain rate between clamped and control studies either before the DSE or at peak stress. Similarly, at 30 min recovery, the HHC was not observed to have any effect on strain or strain rate, although it was associated with a small rise in tissue velocity (Table 3).
Table 3.
Regional wall LV function in studies 1 and 2
| Study 1 | Pre-DSE | Peak stress | Recovery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | HHC | P | Control | HHC | P | Control | HHC | P | |
| Vs (cm/s) | 4.7 ± 1.7 | 4.8 ± 1.8 | 0.10 | 8.5 ± 3.3 | 8.6 ± 3.9 | 0.22 | 4.1 ± 1.6 | 4.6 ± 3.1 | 0.02 |
| Strain (%) | −17.1 ± 6.9 | −16.9 ± 7.7 | 0.46 | −15.8 ± 7.0 | −15.9 ± 6.9 | 0.62 | −14.3 ± 6.9 | −15.3 ± 7.0 | 0.06 |
| Strain rate (s−1) | −1.1 ± 0.4 | −1.1 ± 0.5 | 0.66 | −1.8 ± 0.9 | −1.8 ± 0.8 | 0.89 | −1.0 ± 0.5 | −1.0 ± 0.5 | 0.75 |
| Study 2 | Control | GLP-1 | P | Control | GLP-1 | P | Control | GLP-1 | P |
|---|---|---|---|---|---|---|---|---|---|
| Vs (cm/s) | 4.58 ± 1.62 | 4.56 ± 1.58 | 0.82 | 9.33 ± 3.25 | 9.78 ± 3.21 | 0.008 | 4.46 ± 1.48 | 5.01 ± 1.67 | <0.0001 |
| Strain (%) | −15.18 ± 5.94 | −15.06 ± 5.05 | 0.77 | −15.51 ± 5.39 | −18.09 ± 6.58 | <0.0001 | −14.29 ± 5.35 | −16.62 ± 5.71 | <0.0001 |
| Strain rate (s−1) | −1.04 ± 0.36 | −1.06 ± 0.35 | 0.50 | −2.02 ± 0.71 | −2.30 ± 0.75 | <0.0001 | −0.96 ± 0.31 | −1.18 ± 0.36 | <0.0001 |
Ischemic versus non-ischemic segments
Regions subtended by an artery with a stenosis >70% on coronary angiography were defined as potentially subject to demand ischemia during DSE. The HHC was not observed to have any effect on parameters of regional wall function in either the ischemic or non-ischemic segments at any stage of the study, except for a small rise in tissue velocity in the non-ischemic segments (Table 4).
Table 4.
Ischemic versus non-ischemic segments in study 1
| Ischemic | Pre-DSE | Peak stress | Recovery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | HHC | P | Control | HHC | P | Control | HHC | P | |
| Vs (cm/s) | 4.54 ± 1.7 | 4.66 ± 1.7 | 0.14 | 8.54 ± 3.6 | 8.61 ± 4.7 | 0.44 | 3.94 ± 1.5 | 4.06 ± 1.9 | 0.12 |
| Strain (%) | −16.73 ± 7.0 | −16.68 ± 8.2 | 0.87 | −14.93 ± 7.0 | −15.21 ± 7.1 | 0.49 | −14.33 ± 6.9 | −14.72 ± 6.8 | 0.25 |
| Strain rate (s−1) | −1.02 ± 0.4 | −1.06 ± 0.5 | 0.44 | −1.81 ± 1.0 | −1.84 ± 0.8 | 0.36 | −0.99 ± 0.6 | −1.02 ± 0.5 | 0.32 |
| Non-ischemic | Control | GLP-1 | P | Control | GLP-1 | P | Control | GLP-1 | P |
|---|---|---|---|---|---|---|---|---|---|
| Vs (cm/s) | 4.84 ± 1.8 | 4.95 ± 1.9 | 0.22 | 8.44 ± 3.0 | 8.56 ± 3.1 | 0.23 | 4.34 ± 1.7 | 5.06 ± 3.9 | 0.03 |
| Strain (%) | −17.50 ± 6.8 | −17.22 ± 7.2 | 0.32 | −16.72 ± 7.1 | −16.74 ± 6.8 | 0.77 | −14.24 ± 7.0 | −15.86 ± 7.3 | 0.06 |
| Strain rate (s−1) | −1.12 ± 0.5 | −1.10 ± 0.5 | 0.74 | −1.74 ± 0.7 | −1.78 ± 0.8 | 0.29 | −1.04 ± 0.5 | −1.07 ± 0.5 | 0.23 |
Study 2: effects of GLP-1 (7-36) on myocardial performance during dobutamine stress in the setting of hyperglycemia
Study population
Interim analysis of the first seven patients had shown significant results, and therefore the number of subjects required was reduced. Ten patients were randomly assigned and completed study 2. The clinical characteristics of the subjects are shown in Table 1.
Dobutamine stress echocardiography
The two DSE were conducted 10.8 ± 7.2 days apart. There were no differences in the rate-pressure products at baseline, peak stress or recovery between the two scans (Table 5). Target heart rate was achieved in 6 (60%) subjects in the control HHC study and 5 (50%) in the GLP-1 HHC study. As per the protocol, the dobutamine infusions in the remaining subjects were terminated early due to the development of angina.
Table 5.
Hemodynamic data during DSE scans in study 2
| Peak stress | Recovery | |||||
|---|---|---|---|---|---|---|
| Control-HHC | GLP-1-HHC | P | Control-HHC | GLP-1-HHC | P | |
| Heart rate (bpm) | 126 ± 13 | 125 ± 9 | 0.83 | 71 ± 11 | 77 ± 9 | 0.14 |
| Systolic blood pressure (mm Hg) | 200 ± 25 | 206 ± 25 | 0.82 | 152 ± 22 | 146 ± 29 | 0.37 |
| Diastolic blood pressure (mm Hg) | 84 ± 24 | 81 ± 18 | 0.87 | 83 ± 14 | 84 ± 24 | 0.84 |
| Rate-pressure product (mm Hg bpm) | 25,177 ± 4,564 | 26,197 ± 3,883 | 0.92 | 10,733 ± 2,227 | 11,159 ± 2,788 | 0.54 |
Biochemistry
At baseline, there were no differences in the plasma concentrations of glucose (6.3 ± 0.7 [GLP-1 HHC] versus 6.1 ± 0.7 mmol/l [control HHC], p = 0.36), insulin (73 ± 50 [GLP-1 HHC] versus 71 ± 36 pmol/l [control HHC], p = 0.76), FFA (469 ± 173 [GLP-1 HHC] versus 478 ± 160umol/L [control HHC], p = 0.78) or GLP-1(7-36) (1.8 ± 1.6 [GLP-1 HHC] versus 1.8 ± 1.3 pg/ml [control HHC], p = 0.92) between the GLP-1 and control studies (Fig. 6).
Fig. 6.
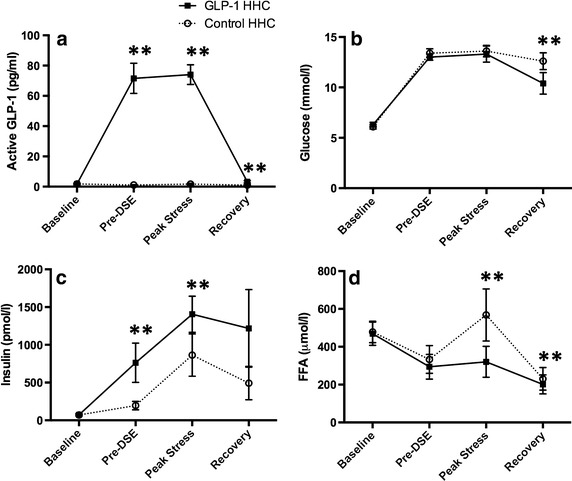
a–d Biochemical data (mean ± SEM) during the DSE scans in study 2 at baseline, pre-DSE, peak stress, and 30-min recovery. **p < 0.05.
As intended, the GLP-1 infusion increased the plasma concentration of active GLP-1(7-36) amide approximately 50 times above the levels in control (pre-DSE 72 ± 28 [GLP-1 HHC] versus 1.1 ± 0.8 pg/ml [control HHC], p = 0.0001; peak stress 74 ± 17 [GLP-1 HHC] versus 1.7 ± 1.4 pg/ml [control HHC], p = 0.0001), and the HHC elevated plasma glucose concentrations during both visits to a similar level (pre DSE 13.0 ± 0.9 [GLP-1 HHC] versus 13.4 ± 1.3 mmol/l [control HHC], p = 0.20; peak stress 13.3 ± 2.4 [GLP-1 HHC] versus 13.6 ± 1.7 mmol/l [control HHC], p = 0.07). However, endogenous insulin production was greater (pre-DSE 763 ± 735 [GLP-1 HHC] versus 195 ± 157 pmol/l [control HHC], p = 0.03; peak stress 1,405 ± 634 [GLP-1 HHC] versus 864 ± 743 pmol/l [control HHC], p = 0.002) and FFA concentrations lower (pre-DSE 302 ± 198 [GLP-1 HHC] versus 359 ± 209umol/l [control HHC], p = 0.006; peak stress 321 ± 216 [GLP-1 HHC] versus 568 ± 364umol/l [control HHC], p = 0.03) after GLP-1 than in the control studies.
In the recovery phase at 30 min after cessation of dobutamine (15 min after cessation of the GLP-1 and glucose infusions), the GLP-1(7-36) amide concentration remained higher than in control (3.2 ± 1.9 [GLP-1 HHC] versus 1.0 ± 1.0 pg/ml [control HHC], p = 0.03) resulting in a lower glucose concentration (10.4 ± 3.2 [GLP-1 HHC] versus 12.6 ± 2.5 mmol/l [control HHC], p = 0.004). Insulin concentrations remained higher (1,218 ± 1,455 [GLP-1 HHC] versus 493 ± 586 pmol/l [control HHC], p = 0.05) and FFA concentrations lower (106 ± 50 [GLP-1 HHC] versus 230 ± 158μmol/l [control HHC], p = 0.02) in the GLP-1 study.
Global LV function
There was no difference in resting EF between the GLP-1 and control studies (pre-DSE 58.1 ± 4.5 [GLP-1 HHC] versus 59.3 ± 6.1% [control HHC], p = 0.41). At peak stress, there was a greater increase in LV function after GLP-1 infusion during hyperglycemia (77.5 ± 5.0 [GLP-1 HHC] versus 71.3 ± 4.3% [control HHC], p = 0.004), and this improved performance persisted into recovery (59.6 ± 3.6 [GLP-1 HHC] versus 50.1 ± 5.8% [control HHC], p < 0.0001). Assessment of global LV function by MASV confirmed these findings. LV function was similar before both scans (pre-DSE 6.1 ± 0.9 [GLP-1 HHC] versus 6.1 ± 0.8 cm/s [control HHC], p = 0.93). However, after GLP-1 infusion during hyperglycemia, myocardial performance was enhanced both at peak stress (11.9 ± 2.7 [GLP-1 HHC] versus 11.0 ± 2.9 cm/s [control HHC], p = 0.01) and at 30 min after dobutamine stress (6.7 ± 0.6 [GLP-1 HHC] versus 6.1 ± 0.7 cm/s [control HHC], p = 0.02) (Fig. 5b).
Regional wall LV function
For the 12 paired non-apical segments, there were no differences in peak systolic tissue velocity, peak systolic strain or peak systolic strain rate between the GLP-1 and control studies pre-DSE. However, at peak dobutamine stress, all three parameters of regional wall function were enhanced after GLP-1 infusion, and these improvements persisted into recovery (Tables 3, 6).
Table 6.
Repeated measures analysis of regional wall function (control versus GLP-1 studies) at different time points in study 2
| Pre-DSE | Peak Stress | Recovery | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean difference | 95% CI of difference | P | Mean difference | 95% CI of difference | P | Mean difference | 95% CI of difference | P | |
| Vs (cm/s) | −0.0002 | −0.29 to 0.29 | >0.99 | −0.42 | −0.80 to −0.04 | 0.02 | −0.56 | −0.86 to −0.26 | <0.0001 |
| Strain (%) | 0.17 | −1.18 to 1.52 | >0.99 | −2.94 | −4.63 to −1.24 | <0.0001 | −2.25 | −3.77 to −0.73 | 0.0006 |
| Strain rate (s−1) | −0.01 | −0.09 to 0.07 | >0.99 | −0.29 | −0.49 to −0.08 | 0.001 | −0.23 | −0.32 to −0.13 | <0.0001 |
Ischemic vs non-ischemic segments
GLP-1 infusion during hyperglycemia had a greater beneficial effect on ischemic than on non-ischemic segments (Table 7).
Table 7.
Ischemic versus non-ischemic segments in study 2
| Ischaemic | Pre-DSE | Peak stress | Recovery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | HHC | P | Control | HHC | P | Control | HHC | P | |
| Vs (cm/s) | 4.69 ± 1.53 | 4.66 ± 1.48 | 0.46 | 8.50 ± 3.07 | 8.70 ± 2.99 | 0.03 | 4.59 ± 1.53 | 4.90 ± 1.79 | <0.0001 |
| Strain (%) | −14.84 ± −5.51 | −14.91 ± 4.39 | 0.82 | −16.75 ± 5.26 | −18.26 ± 7.38 | <0.0001 | −15.61 ± 5.47 | −16.65 ± 5.72 | 0.006 |
| Strain rate (s−1) | −0.99 ± 0.29 | −1.00 ± 0.29 | 0.71 | −2.02 ± 0.79 | −2.17 ± 0.70 | <0.0001 | −1.10 ± 0.29 | −1.21 ± 0.38 | <0.0001 |
| Non-ischaemic | Control | GLP-1 | P | Control | GLP-1 | P | Control | GLP-1 | P |
|---|---|---|---|---|---|---|---|---|---|
| Vs (cm/s) | 4.44 ± 1.73 | 4.46 ± 1.70 | 0.81 | 10.80 ± 3.05 | 11.06 ± 3.01 | 0.09 | 4.92 ± 1.41 | 5.15 ± 1.52 | 0.001 |
| Strain (%) | −15.43 ± 6.41 | −15.23 ± 5.75 | 0.48 | −16.86 ± 5.59 | −17.89 ± 5.57 | 0.007 | −15.26 ± 5.27 | −16.57 ± 5.79 | 0.0009 |
| Strain rate (s−1) | −1.11 ± 0.41 | −1.12 ± 0.39 | 0.55 | −2.32 ± 0.70 | −2.45 ± 0.79 | 0.006 | −1.04 ± 0.32 | −1.15 ± 0.35 | <0.0001 |
Reproducibility
We have previously assessed the reproducibility of the parameters presented in six randomly selected patients for the images recorded at rest, peak stress, and in recovery. The intra- and inter-observer variations for the tissue Doppler imaging parameters were, respectively, 6.12 ± 0.96% and 6.40 ± 0.99% for MASV, 7.22 ± 0.93% and 5.80 ± 0.76% for tissue velocity, 11.0 ± 3.56% and 12.6 ± 4.04% for strain, and 9.75 ± 0.29% and 11.6 ± 0.34% for strain rate. For LVEF, the intra-observer variation was 5.69 ± 7.00%, and the inter-observer variation was 6.59 ± 8.18%.
Discussion
In these studies, we have demonstrated two key findings in patients with IHD: (1) HH has a neutral effect on LV function during dobutamine stress; and (2) in patients with T2DM, an intravenous infusion of GLP-1(7-36) amide at the time of hyperglycemia enhances myocardial performance during dobutamine stress.
Hyperglycemia and the myocardium
It is well recognised that acute hyperglycemia may have a direct detrimental effect on ischemic myocardium. Although a large number of potential mechanisms have been postulated, those relevant to demand ischemia include increased oxidative stress and reduced nitric oxide production [23] leading to a reduction in flow-mediated, endothelium-dependent vasodilatation [24, 25], and increased production of endothelial vasoconstrictor prostanoids which impairs the acetylcholine-mediated vasodilator response [26]. In contrast, hyperinsulinemia is thought to have several beneficial effects on ischaemic myocardium, including a reduction in the circulating concentration of FFAs [27], and a subsequent shift towards greater myocardial glucose utilization, which improves overall myocardial oxygen efficiency [28].
In our study, the HHC stimulated endogenous insulin production and resulted in inhibition of lipolysis with an associated reduction in FFA concentration. At peak stress, the combination of pancreatic β1-stimulation from dobutamine and hyperglycemia led to a marked potentiation of insulin release, increasing further the differences between insulin and FFA. These metabolic differences persisted (but to a lesser degree) in the recovery phase at 30 min after cessation of dobutamine. However, despite these metabolic alterations, there was no significant net effect observed on either global or regional LV function during dobutamine stress or in recovery.
These findings are in contrast to the results of our previous work where we observed improved myocardial performance in patients with CAD undergoing DSE during the steady state stage of a HEC [17]. Whilst both clamp techniques would be expected to promote similar changes in myocardial metabolism (i.e. a transition from predominantly FFA oxidation to greater glucose utilization), HHC has been shown to reduce myocardial perfusion reserve when compared with HEC in both diabetic [29] and non-diabetic patients [30]. Therefore, the observed differences on LV function between the two clamp techniques might be explained by a counterbalance between the favourable effects of hyperinsulinemia on myocardial function and the inhibitory effects of hyperglycemia on coronary vasodilatation. In contrast to this hypothesis, Nielsen et al. found that short-term hyperglycemia (induced by discontinuation of insulin) in patients with T2DM increased LV contractility in patients with both normal and reduced EF. However, these results are not directly comparable to those of our study, which assessed non-insulin requiring diabetics using a different protocol to induce hyperglycemia (i.e. exogenous glucose clamp with subsequent large increase in insulin levels) [31].
Insulin resistance and the myocardium
Insulin resistance refers to the reduced response of glucose uptake to the stimulatory effect of insulin. In the myocardium, insulin resistance suppresses oxidation of glucose, improves fatty acid metabolism and alters intracellular signalling, leading to numerous impairments of excitation–contraction coupling, less efficient energy production and increased susceptibility to ischemia/reperfusion injury [32]. Although these changes are common in patients with diabetes, they are frequently unrecognised. However, sensitive echocardiographic techniques such as TDI and SR imaging allow for earlier detection of asymptomatic LV dysfunction in these individuals. Compared with healthy age- and sex-matched controls, subjects with insulin resistance demonstrate a reduced increase in longitudinal SR values during DSE, suggesting reduced LV contractile reserve [33]. In patients with diabetes, longitudinal strain reserve (defined as the difference between global longitudinal strain before and after dipyridamole infusion) is increased compared to non-diabetics [34]. Furthermore, patients with T2DM and microvascular angina demonstrate reduced early diastolic peak velocities and peak systolic two-dimensional strain than healthy age- and sex-matched controls [35].
GLP-1 and hyperglycemia
To the best of our knowledge, our study is the first to investigate the effect of GLP-1 modulation on LV function during myocardial ischemia in the setting of hyperglycemia in patients with T2DM and CAD. In study 2, there were no differences in GLP-1 levels or LV function between the two scans at baseline. Infusion of GLP-1 during HHC increased the plasma concentration of active GLP-1(7-36) amide approximately 50 times above the levels in control, resulting in increased insulin and suppressed FFA concentrations. Compared with control, GLP-1 was associated with improved parameters of both global and regional LV performance at peak stress and at 30 min recovery. These effects were predominantly driven by a reduction in contractile dysfunction in regions subject to demand ischemia. Glucose levels were similarly elevated in both scans, suggesting that the favorable cardiovascular effects were due to GLP-1 itself and not to euglycemia. Although insulin concentrations were higher during the GLP-1 scan, numerous studies have demonstrated that incretin-related cardioprotection occurs independent of an effect on insulin [14, 16, 36].
Over the last decade, there has been an increasing body of evidence highlighting the favorable cardiovascular properties of GLP-1 [37–39]. In animal studies that have assessed cardiac metabolic alterations, GLP-1 modulation was associated with increased myocardial glucose uptake [40, 41], reduced myocardial levels of lactate and pyruvate [42], and an increase in the relative oxidation of carbohydrate versus fat [41]. GLP-1 may therefore confer its cardioprotective effects by inducing a shift towards greater myocardial glucose utilization, an adaptive response which is known to be more oxygen efficient than fatty acid metabolism [43], but which is impaired in the context of insulin resistance. In keeping with this concept, we found that ischemic segments derive more benefit than non-ischemic segments.
Hyperglycaemia is known to be associated with an adverse prognosis in patients presenting with ACS [1]. However, attempts to improve outcomes with insulin-based strategies have proven to be ineffective. Intense insulin therapy (to achieve euglycemia) has been associated with increased mortality and hypoglycemia when compared with moderate glucose control [4]. Similarly, glucose-insulin-potassium infusions (which aim to achieve high insulin and circulating glucose levels in order to increase myocardial glucose uptake and inhibit the adverse effects of FFA) have also failed to demonstrate consistent clinical benefits [5]. The results of our study indicate that GLP-1 may offer an important therapeutic adjunct as a metabolic agent for cardioprotection in patients with T2DM.
Limitations
This study has a number of important limitations. Firstly, we have used tissue Doppler-derived indices of myocardial deformation to assess regional LV function, which has a number of potential pitfalls compared with two-dimensional speckle tracking echocardiography (STE). These include a high dependence on a favorable angle of incidence during image acquisition, an inability to accurately assess apical segments, and potentially high inter-and intra-observer variations. However, since TDI allows for measurements of strain and strain rate with excellent temporal resolution, this technique may be advantageous to STE at times of higher heart rates (e.g. during dobutamine stress). Secondly, whilst all attempts were made to conduct the two DSE scans for each patient in identical fashion and to obtain the peak stress images at the same degree of dobutamine stress, we cannot exclude the possibility of a degree of variation in the response of individual patients to dobutamine on the two separate study days. Thirdly, the potential cardioprotective effects of GLP-1 have been assessed using echocardiographic (and not clinical) endpoints in only a small number of patients. Further work, in the form of adequately powered randomized controlled trials, is required to ascertain if these favorable cardiovascular effects at the time of demand ischemia translate into an improvement in clinical outcomes.
Conclusions
In patients with obstructive CAD, hyperglycemic hyperinsulinemia has a neutral effect on LV function during dobutamine stress. However, an infusion of GLP-1(7-36) at the time of hyperglycemia protects the heart against demand ischemic LV systolic dysfunction in patients with T2DM and IHD.
Authors’ contributions
Conception and design—LMM, PMH, DPD. Acquisition, analysis and interpretation of data—LMM, PMH, LSR, ACK, SJC, SPH, DPD. Drafting the manuscript—LMM, PMH, LSR, ACK, SJC. Revision of the manuscript for important intellectual content—SPH, DPD. All authors read and approved the final manuscript.
Acknowledgements
We thank the patients for their participation and the staff at the Wellcome Trust Clinical Research Facility, Addenbrooke’s Hospital, for their assistance throughout the study.
Funding sources
This study was funded by the Medical Research Council (London, UK) and supported by the Cambridge NIHR Comprehensive Biomedical Research Centre.
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Abbreviations
- ACS
acute coronary syndromes
- FFA
free fatty acids
- GLP-1
glucagon-like peptide-1
- IHD
ischaemic heart disease
- HEC
hyperinsulinemic euglycemic clamp
- DSE
dobutamine stress echocardiography
- CAD
coronary artery disease
- LV
left ventricular
- HH
hyperinsulinemic hyperglycemia
- T2DM
type 2 diabetes mellitus
- EF
ejection fraction
- DPP4
dipeptidyl peptidase-4
- HHC
hyperinsulinemic hyperglycemic clamp
- TDI
tissue Doppler imaging
- MASV
mitral annular systolic velocity
- STE
speckle tracking echocardiography
Contributor Information
Liam M McCormick, Email: drliammccormick@doctors.org.uk.
Patrick M Heck, Email: pmheck@doctors.org.uk.
Liam S Ring, Email: liamring@doctors.org.uk.
Anna C Kydd, Email: annakydd@doctors.org.uk.
Sophie J Clarke, Email: sophie.j.clarke@gmail.com.
Stephen P Hoole, Email: stevieh@doctors.org.uk.
David P Dutka, Email: dpd24@medschl.cam.ac.uk.
References
- 1.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 2.Deedwania P, Kosiborod M, Barrett E, Ceriello A, Isley W, Mazzone T, et al. Hyperglycemia and acute coronary syndrome: a scientific statement from the American Heart Association Diabetes Committee of the Council on Nutrition, physical activity, and metabolism. Circulation. 2008;117:1610–1619. doi: 10.1161/CIRCULATIONAHA.107.188629. [DOI] [PubMed] [Google Scholar]
- 3.Opie LH. Metabolic management of acute myocardial infarction comes to the fore and extends beyond control of hyperglycemia. Circulation. 2008;117:2172–2177. doi: 10.1161/CIRCULATIONAHA.108.780999. [DOI] [PubMed] [Google Scholar]
- 4.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, Blair D, Foster D. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 5.Zhao YT, Weng CL, Chen ML, Li KB, Ge YG, Lin XM, et al. Comparison of glucose-insulin-potassium and insulin-glucose as adjunctive therapy in acute myocardial infarction: a contemporary meta-analysis of randomised controlled trials. Heart. 2010;96:1622–1626. doi: 10.1136/hrt.2010.194563. [DOI] [PubMed] [Google Scholar]
- 6.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr, Ganiats TG, Holmes DR, Jr, et al. 2014 AHA/ACC Guideline for the management of patients with non-st-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:2645–2687. doi: 10.1016/j.jacc.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 7.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, et al. ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;2013(127):e362–e425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 8.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 9.Mehta SR, Yusuf S, Diaz R, Zhu J, Pais P, Xavier D, et al. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. JAMA. 2005;293:437–446. doi: 10.1001/jama.293.4.437. [DOI] [PubMed] [Google Scholar]
- 10.Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 11.Lonborg J, Vejlstrup N, Kelbaek H, Botker HE, Kim WY, Mathiasen AB, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012;33:1491–1499. doi: 10.1093/eurheartj/ehr309. [DOI] [PubMed] [Google Scholar]
- 12.Read PA, Hoole SP, White PA, Khan FZ, O’Sullivan M, West NE, et al. A pilot study to assess whether glucagon-like peptide-1 protects the heart from ischemic dysfunction and attenuates stunning after coronary balloon occlusion in humans. Circ Cardiovasc Interv. 2011;4:266–272. doi: 10.1161/CIRCINTERVENTIONS.110.960476. [DOI] [PubMed] [Google Scholar]
- 13.Read PA, Khan FZ, Dutka DP. Cardioprotection against ischaemia induced by dobutamine stress using glucagon-like peptide-1 in patients with coronary artery disease. Heart. 2012;98:408–413. doi: 10.1136/hrt.2010.219345. [DOI] [PubMed] [Google Scholar]
- 14.Read PA, Khan FZ, Heck PM, Hoole SP, Dutka DP. DPP-4 inhibition by sitagliptin improves the myocardial response to dobutamine stress and mitigates stunning in a pilot study of patients with coronary artery disease. Circ Cardiovasc Imaging. 2010;3:195–201. doi: 10.1161/CIRCIMAGING.109.899377. [DOI] [PubMed] [Google Scholar]
- 15.McCormick LM, Kydd AC, Read PA, Ring LS, Bond SJ, Hoole SP, et al. Chronic dipeptidyl peptidase-4 inhibition with sitagliptin is associated with sustained protection against ischemic left ventricular dysfunction in a pilot study of patients with type 2 diabetes mellitus and coronary artery disease. Circulation Cardiovascu Imaging. 2014;7:274–281. doi: 10.1161/CIRCIMAGING.113.000785. [DOI] [PubMed] [Google Scholar]
- 16.McCormick LM, Hoole SP, White PA, Read PA, Axell RG, Clarke SJ, et al. Pre-treatment with glucagon-like peptide-1 protects against ischemic left ventricular dysfunction and stunning without a detected difference in myocardial substrate utilization. JACC Cardiovasc Interv. 2015;8:292–301. doi: 10.1016/j.jcin.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Heck PM, Hoole SP, Khan SN, Dutka DP. Hyperinsulinemia improves ischemic LV function in insulin resistant subjects. Cardiovasc Diabetol. 2010;9:27. doi: 10.1186/1475-2840-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 19.Pellerin D, Sharma R, Elliott P, Veyrat C. Tissue Doppler, strain, and strain rate echocardiography for the assessment of left and right systolic ventricular function. Heart. 2003;89(Suppl 3):iii9–iii17. doi: 10.1136/heart.89.suppl_3.iii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madler CF, Payne N, Wilkenshoff U, Cohen A, Derumeaux GA, Pierard LA, et al. Non-invasive diagnosis of coronary artery disease by quantitative stress echocardiography: optimal diagnostic models using off-line tissue Doppler in the MYDISE study. Eur Heart J. 2003;24:1584–1594. doi: 10.1016/S0195-668X(03)00099-X. [DOI] [PubMed] [Google Scholar]
- 21.Voigt JU, Exner B, Schmiedehausen K, Huchzermeyer C, Reulbach U, Nixdorff U, et al. Strain-rate imaging during dobutamine stress echocardiography provides objective evidence of inducible ischemia. Circulation. 2003;107:2120–2126. doi: 10.1161/01.CIR.0000065249.69988.AA. [DOI] [PubMed] [Google Scholar]
- 22.Voigt JU, Nixdorff U, Bogdan R, Exner B, Schmiedehausen K, Platsch G, et al. Comparison of deformation imaging and velocity imaging for detecting regional inducible ischaemia during dobutamine stress echocardiography. Eur Heart J. 2004;25:1517–1525. doi: 10.1016/j.ehj.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Tesfamariam B, Brown ML, Deykin D, Cohen RA. Elevated glucose promotes generation of endothelium-derived vasoconstrictor prostanoids in rabbit aorta. J Clin Invest. 1990;85:929–932. doi: 10.1172/JCI114521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawano H, Motoyama T, Hirashima O, Hirai N, Miyao Y, Sakamoto T, et al. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J Am Coll Cardiol. 1999;34:146–154. doi: 10.1016/S0735-1097(99)00168-0. [DOI] [PubMed] [Google Scholar]
- 25.Shige H, Ishikawa T, Suzukawa M, Ito T, Nakajima K, Higashi K, et al. Endothelium-dependent flow-mediated vasodilation in the postprandial state in type 2 diabetes mellitus. Am J Cardiol. 1999;84(1272–4):A9. doi: 10.1016/s0002-9149(99)00548-2. [DOI] [PubMed] [Google Scholar]
- 26.Tesfamariam B, Cohen RA. Role of superoxide anion and endothelium in vasoconstrictor action of prostaglandin endoperoxide. Am J Physiol. 1992;262:H1915–H1919. doi: 10.1152/ajpheart.1992.262.6.H1915. [DOI] [PubMed] [Google Scholar]
- 27.Ferrannini E, Galvan AQ, Gastaldelli A, Camastra S, Sironi AM, Toschi E, et al. Insulin: new roles for an ancient hormone. Eur J Clin Invest. 1999;29:842–852. doi: 10.1046/j.1365-2362.1999.00536.x. [DOI] [PubMed] [Google Scholar]
- 28.Ferrannini E, Santoro D, Bonadonna R, Natali A, Parodi O, Camici PG. Metabolic and hemodynamic effects of insulin on human hearts. Am J Physiol. 1993;264:E308–E315. doi: 10.1152/ajpendo.1993.264.2.E308. [DOI] [PubMed] [Google Scholar]
- 29.Srinivasan M, Herrero P, McGill JB, Bennik J, Heere B, Lesniak D, et al. The effects of plasma insulin and glucose on myocardial blood flow in patients with type 1 diabetes mellitus. J Am Coll Cardiol. 2005;46:42–48. doi: 10.1016/j.jacc.2005.03.056. [DOI] [PubMed] [Google Scholar]
- 30.Abdelmoneim SS, Hagen ME, Mendrick E, Pattan V, Wong B, Norby B, et al. Acute hyperglycemia reduces myocardial blood flow reserve and the magnitude of reduction is associated with insulin resistance: a study in nondiabetic humans using contrast echocardiography. Heart Vessels. 2013;28:757–768. doi: 10.1007/s00380-012-0305-y. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen R, Norrelund H, Kampmann U, Botker HE, Moller N, Wiggers H. Effect of acute hyperglycemia on left ventricular contractile function in diabetic patients with and without heart failure: two randomized cross-over studies. PLoS One. 2013;8:e53247. doi: 10.1371/journal.pone.0053247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miki T, Yuda S, Kouzu H, Miura T. Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev. 2013;18:149–166. doi: 10.1007/s10741-012-9313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cadeddu C, Nocco S, Piano D, Deidda M, Cossu E, Baroni MG, et al. Early impairment of contractility reserved in patients with insulin resistance in comparison with health subjects. Cardiovasc Diabetol. 2013;12:66. doi: 10.1186/1475-2840-12-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cognet T, Vervueren PL, Dercle L, Bastie D, Richaud R, Berry M, et al. New concept of myocardial longitudinal strain reserve assessed by a dipyridamole infusion using 2D-strain echocardiography: the impact of diabetes and age, and the prognostic value. Cardiovasc Diabetol. 2013;12:84. doi: 10.1186/1475-2840-12-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Andrea A, Nistri S, Castaldo F, Galderisi M, Mele D, Agricola E, et al. Working group nucleus on echocardiography of Italian society of cardiology. Int J Cardiol. 2012;154:250–255. doi: 10.1016/j.ijcard.2010.09.044. [DOI] [PubMed] [Google Scholar]
- 36.Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen YT, et al. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317:1106–1113. doi: 10.1124/jpet.106.100982. [DOI] [PubMed] [Google Scholar]
- 37.Zhao TC. Glucagon-like peptide-1 (GLP-1) and protective effects in cardiovascular disease: a new therapeutic approach for myocardial protection. Cardiovasc Diabetol. 2013;12:90. doi: 10.1186/1475-2840-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clarke SJ, McCormick LM, Dutka DP. Optimising cardioprotection during myocardial ischaemia: targeting potential intracellular pathways with glucagon-like peptide-1. Cardiovasc Diabetol. 2014;13:12. doi: 10.1186/1475-2840-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCormick LM, Kydd AC, Dutka DP. Cardiac protection via metabolic modulation: an emerging role for incretin-based therapies? Cardiovasc Hematol Agents Med Chem. 2012;10:319–324. doi: 10.2174/187152512803530360. [DOI] [PubMed] [Google Scholar]
- 40.Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, et al. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110:955–961. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- 41.Bao W, Aravindhan K, Alsaid H, Chendrimada T, Szapacs M, Citerone DR, et al. Albiglutide, a long lasting glucagon-like peptide-1 analog, protects the rat heart against ischemia/reperfusion injury: evidence for improving cardiac metabolic efficiency. PLoS One. 2011;6:e23570. doi: 10.1371/journal.pone.0023570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kavianipour M, Ehlers MR, Malmberg K, Ronquist G, Ryden L, Wikstrom G, et al. Glucagon-like peptide-1 (7-36) amide prevents the accumulation of pyruvate and lactate in the ischemic and non-ischemic porcine myocardium. Peptides. 2003;24:569–578. doi: 10.1016/S0196-9781(03)00108-6. [DOI] [PubMed] [Google Scholar]
- 43.Liedtke AJ. Alterations of carbohydrate and lipid metabolism in the acutely ischemic heart. Prog Cardiovasc Dis. 1981;23:321–336. doi: 10.1016/0033-0620(81)90019-0. [DOI] [PubMed] [Google Scholar]


