Abstract
Here, we report a novel social orienting response that occurs after viewing averted gaze. We show, in three experiments, that when a person looks from one location to an object, attention then shifts towards the face of an individual who has subsequently followed the person's gaze to that same object. That is, contrary to ‘gaze following’, attention instead orients in the opposite direction to observed gaze and towards the gazing face. The magnitude of attentional orienting towards a face that ‘follows’ the participant's gaze is also associated with self-reported autism-like traits. We propose that this gaze leading phenomenon implies the existence of a mechanism in the human social cognitive system for detecting when one's gaze has been followed, in order to establish ‘shared attention’ and maintain the ongoing interaction.
Keywords: joint attention, shared attention, gaze perception, social orienting, social cognition
1. Introduction
Humans, among other species, spontaneously follow the gaze direction of other individuals to orient their own attention towards a common object—establishing ‘joint attention’ [1]. The attentional underpinnings of joint attention can be studied with the gaze cueing paradigm, which has shown that orienting in the direction in which someone else is looking appears to occur in a rapid and robust manner (e.g. [2,3]; see [4] for review). Following gaze to establish joint attention is beneficial to the ‘follower’ as they may learn about important stimuli, infer the mental state of others and predict their future actions (e.g. [5]).
Where there is a follower, there is also a ‘leader’, who has effected change on their social environment. A great deal of research has focused on investigating how we code others' gaze direction (e.g. [6]). In dynamic interactions, however, it is also important to detect the effects that our own gaze behaviour has on others [7], and relatively less is known about how this is achieved. Therefore, to understand the mechanisms underpinning joint attention more completely, we need to consider the ‘initiator’, in addition to the ‘responder’ [8,9]. Some intriguing findings regarding gaze leading have recently been uncovered using gaze-contingent paradigms so that face stimuli ‘respond’ to the participant's gaze behaviour, showing that ‘being followed’ has consequences for various components of joint attention (e.g. [10,11]). For example, gaze leading positively influences affective processing [12–14] and recognition memory for gaze leaders [15]. It is likely that gaze coding mechanisms, spatial attention and social cognitive systems could be involved in detecting and maintaining iterative social orienting behaviours such as when one's eyes are followed by a conspecific [7,16–18].
Here we focus on elucidating the putative attention mechanisms engaged when one detects that one's gaze has been followed. It is reasonable to hypothesize the existence of a mechanism that emerged to potentiate beneficial social interactions by driving the detection of such positive interactions. In a sense, when our eyes are followed, another individual has ‘imitated’ our attentional state. Imitation is crucial for the development of social cognition [19], and many primate species recognize being imitated [20,21] (see also [22]). It is possible that having your gaze followed could be similarly salient. Therefore, here we ask whether causing another individual to change their attentional state to one of joint attention has an effect on spatial attention in the initiator. We hypothesize that the gaze leader—once followed—will shift their attention towards the follower. Discovering such a response would imply a mechanism that drives humans to establish a state of ‘shared attention’ [23]. Shared attention is where one individual follows another, but additionally, both individuals are aware of their common attentional focus. Shared attention is therefore a more elaborate, reciprocal, joint attention episode that is thought to be expressed only in humans [24–26], and may play a particularly crucial role in language acquisition [27].
To determine whether an orienting mechanism facilitates shared attention, we conducted a series of computer-based laboratory experiments in which we assessed attention shifts towards peripherally presented faces. In each experimental trial, the participant would look from a fixation point in the lower portion of a display to an image of a real-world object located centrally. Our design therefore incorporates two important aspects of joint attention. First, one agent actively re-orients attention elsewhere to gain visual information, and second, this eye movement foveates a meaningful object [28]. The latter object-based nature of joint attention is required by definition [23] and is critical in human social development [29]. Then, one face would look at the location to which the participant had looked and another face would look in the opposite direction. By comparing manual reaction times (RTs) to discriminate targets appearing on these faces, we could assess whether a face that follows the participant's eyes captures attention.
2. Experiment 1
Participants responded to targets appearing in four possible locations: on two faces to the immediate left and right of the centrally presented object, and two other faces presented more peripherally (aligned horizontally; figure 1). After the participants had moved their eyes from the lower fixation cross to the central object, the two innermost faces would simultaneously open their eyes and look in a common direction (left or right), meaning one face looks towards the central object while the other looks away. A target letter would appear on the bridge of the nose of one of the four faces. The two innermost faces are the critical target loci for evaluating our hypothesis, but performance at the peripheral loci could also be informative. Our hypothesis is that participants will orient towards a face that suddenly looks at the location to which they are currently looking, and respond to targets appearing there more quickly than at any other location.
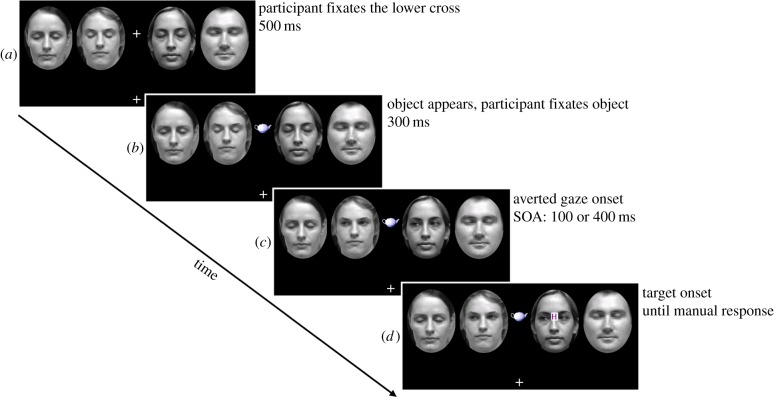
Figure 1.
Example trial from experiment 1, where the target (here letter H) has appeared on the joint attention face. (a) Participants were first asked to look at the lower cross for 500 ms. (b) After that, the upper cross was replaced with an object and participants were asked to look at the object for 300 ms. (c) Then, the two innermost faces were shown with averted gaze in a common direction for either 100 or 400 ms, depending on SOA. (d) Finally, a target letter (N or H) would appear on one of the faces until identification (or until timeout—5000 ms). A centrally presented red cross would follow response on incorrect trials. Targets could appear on the bridge of the nose of any one of the faces. (Online version in colour.)
(a). Method
In all experiments, we have reported how we determined our sample size, all data exclusions (if any), all manipulations and all measures we have collected [30].
(i). Participants
Thirty-two adults (mean age = 19.2 years, s.d. = 1.6 years; eight males) took part in return for course credit or payment (as in this experiment and in all others). Owing to calibration and tracking difficulties, three participants completed only three of four blocks. Participants in all experiments reported normal or corrected-to-normal vision. We aimed to collect data from approximately 30 participants and stopped at n = 32, for convenience, at the end of a block of booked testing sessions.
(ii). Apparatus and stimuli
Face stimuli consisted of greyscale photographs taken from an existing stimulus set [31]. Eight identities with a calm facial expression were used (four female). For each face identity, three images were used such that each identity could be displayed with closed eyes, leftward gaze or rightward gaze. Each face image measured 50 × 64 mm. Two faces appeared on each side of the central cross; the centre-point of the innermost faces was 33 mm away from the centre, and the centre-point of the outermost faces was 84 mm away from the centre. Participants began each trial fixating a second cross 65 mm directly below the central cross. Face identities were randomly assigned to each position on each trial, with the constraint that a given identity could only appear in one location per trial. The referent object of the joint attention episode was one of 16 not-to-scale images of objects commonly found in the kitchen, randomly selected on each trial [32]. Target letters (N or H; 18 pt purple bold Arial, 4 × 4 mm) were presented on a white background (6 × 6 mm). Stimulus presentation was controlled by a standard desktop computer with a 46 cm screen (1024 × 768 px), and manual responses were made on a standard keyboard. Participants were positioned comfortably in a chin-rest. Right eye position was recorded (Eyelink 1000, SR Research, Ontario, Canada; spatial resolution 0.1°, 500 Hz). In this and subsequent experiments, the Autism Spectrum Quotient (AQ) questionnaire was used to measure autism-like traits of participants [33], which has previously been shown to share a relationship with social orienting of attention [34–36].
(iii). Design
A 4 (target location: ‘face with eyes closed and looked away from’, ‘joint attention face’, ‘non-joint attention face’, ‘face with eyes closed and being looked at’) × 2 (cue-target stimulus onset asynchrony—‘SOA’: 100 ms, 400 ms) within-subjects design was used. Although we used four target locations, the two critical target locations are the innermost ones (‘joint attention face’ and ‘non-joint attention face’). We used the additional target locations to assess how attention was distributed beyond these two locations. RTs and accuracy rates were measured.
(iv). Procedure
Participants sat approximately 70 cm away from the display. Each trial started with the two fixation crosses (central, and 65 mm below) along with the four faces aligned on the horizontal midline, two either side of the central cross (figure 1a), on a black background. On each trial, the participant first had to fixate the lower cross for 500 ms, which would trigger the replacement of the central cross with an image of an object, which was their cue to saccade to and fixate the object. After fixating the object for 300 ms, the innermost faces were then both displayed with averted gaze in a common direction (left or right) for either 100 or 400 ms SOA prior to target appearance (figure 1c). Thus, for example, the face to the right of fixation would ‘look’ leftwards towards the central object, while the face to the left of fixation, also looking leftwards, looked away from the object. Next, a target letter was presented on the bridge of the nose of one of the four faces until a response was made, or until timeout (5000 ms), whichever came first. Note that although participants were asked to fixate the central object in order to progress through each trial, they were given no specific instructions regarding maintaining fixation following cue or target appearance. Participants responded to targets with their preferred index finger on the H key for ‘H’ targets and with the thumb of the same hand on the spacebar for ‘N’ targets. Speed and accuracy of manual response was emphasized. Finally, a feedback screen was displayed for 1500 ms—this was a black screen after a correct response, but showed a red central cross after an incorrect or non-response. The experimenter was present for the duration of the session. Female experimenter L.J.S. tested 18 participants; male experimenter S.G.E. tested 15 participants. The experiment comprised 256 trials (split evenly into four blocks) and the session took approximately 60 min.
(b). Results and discussion
Mean and standard deviations for accuracy and RTs for each condition (and all experiments) are found in table 1. Trials with correct RTs 3 s.d. above or below the participant's mean were removed before the calculation of means for each condition. The same criteria were used in each of the three experiments.
Table 1.
Mean and s.d. (in parentheses) for reaction times (RT; ms) and accuracy (%) in all conditions and the gaze leading effect magnitude (ms; RT at Non-Joint Attention face minus RT at Joint Attention face. Note: positive numbers indicate a bias towards the Joint Attention face) in Experiments 1–3. Face types are referred to as: N, Not looked at; JA, Joint Attention; nJA, Non-Joint Attention; L, Looked at by both faces.
| SOA 100 ms |
SOA 400 ms |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | JA | nJA | L | N | JA | nJA | L | ||
| experiment 1 | RT | 666 (121) | 612 (110) | 631 (123) | 676 (119) | 616 (177) | 582 (118) | 601 (125) | 643 (125) |
| accuracy | 95.4 (5.0) | 95.2 (4.6) | 96.2 (3.9) | 96.1 (3.5) | 96.7 (3.5) | 96.5 (4.6) | 95.6 (4.2) | 96.7 (4.2) | |
| experiment 2 | RT | 642 (146) | 653 (146) | 589 (133) | 605 (126) | ||||
| accuracy | 97.1 (2.5) | 96.2 (4.2) | 98.2 (2.9) | 97.3 (2.7) | |||||
| experiment 3 | RT | 613 (126) | 623 (129) | 566 (124) | 576 (131) | ||||
| accuracy | 95.1 (4.3) | 97.0 (4.3) | 96.6 (4.2) | 96.0 (4.1) | |||||
(i). Accuracy
A 4 (target location: joint attention, non-joint attention, looked at, not looked at) × 2 (SOA: 100 ms, 400 ms) repeated-measures ANOVA was carried out on mean accuracy (96.0% of trials, s.d. = 3.51%). There was no significant main effect of target location (F3,93 < 1, SOA, F1,31 = 3.62, p = 0.066, 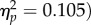 and no significant interaction (F3,93 = 1.03, p = 0.38,
and no significant interaction (F3,93 = 1.03, p = 0.38, 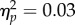 ).
).
(ii). Reaction times
A total of 1.7% of trials were discarded as outliers. A 4 (target location: joint attention, non-joint attention, looked at, not looked at) × 2 (SOA: 100 ms, 400 ms) repeated-measures ANOVA showed that the main effect of target location was significant (F3,93 = 49.6, p < 0.001, 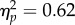 ). There was a significant main effect of SOA (F1,31 = 52.0, p < 0.001,
). There was a significant main effect of SOA (F1,31 = 52.0, p < 0.001, 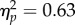 ). The interaction was not significant (F3,93 = 1.86, p = 0.14,
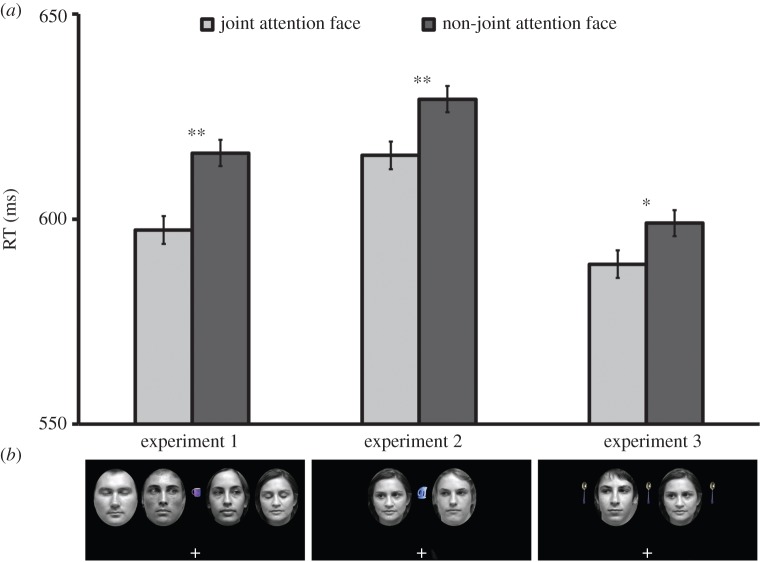
). The interaction was not significant (F3,93 = 1.86, p = 0.14, 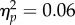 ). To determine the source of the target location main effect, a 2 (target location: joint attention, non-joint attention) × 2 (SOA: 100 ms, 400 ms) repeated-measures ANOVA of the inner locations showed that—as predicted—RTs at the joint attention face target location (597 ms) were faster than at the non-joint attention face (616 ms; F1,31 = 19.0, p < 0.001,
). To determine the source of the target location main effect, a 2 (target location: joint attention, non-joint attention) × 2 (SOA: 100 ms, 400 ms) repeated-measures ANOVA of the inner locations showed that—as predicted—RTs at the joint attention face target location (597 ms) were faster than at the non-joint attention face (616 ms; F1,31 = 19.0, p < 0.001, 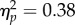 ; figure 2). This effect was reliable at the 100 ms SOA (t31 = −3.45, p = 0.002, dz = 0.61) and the 400 ms SOA (t31 = −2.60, p = 0.014, dz = 0.46). Additionally, the main effect of SOA was significant (F1,31 = 17.37, p < 0.001,
; figure 2). This effect was reliable at the 100 ms SOA (t31 = −3.45, p = 0.002, dz = 0.61) and the 400 ms SOA (t31 = −2.60, p = 0.014, dz = 0.46). Additionally, the main effect of SOA was significant (F1,31 = 17.37, p < 0.001, 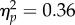 ) and the interaction was non-significant (F1,31 < 1). The same 2 × 2 ANOVA for peripheral locations revealed faster RTs at the ‘not looked at’ face than at the ‘looked at’ face (F1,31 = 9.02, p
= 0.005,
) and the interaction was non-significant (F1,31 < 1). The same 2 × 2 ANOVA for peripheral locations revealed faster RTs at the ‘not looked at’ face than at the ‘looked at’ face (F1,31 = 9.02, p
= 0.005, 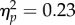 ). The SOA main effect was significant (F1,31 = 72.76, p < 0.001,
). The SOA main effect was significant (F1,31 = 72.76, p < 0.001, 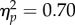 ) and the interaction was not (F1,31 = 3.60, p
= 0.067,
) and the interaction was not (F1,31 = 3.60, p
= 0.067, 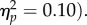
Figure 2.
(a) Graphs show RTs for critical target locations in experiments 1–3. Error bars are within-subject standard error of the mean [37] from the main effect term in the 2 × 2 ANOVA performed on performance at these locations. Asterisks denote statistically significant effects, **p < 0.01, *p < 0.05 (see also table 1). (b) Examples of stimulus displays immediately prior to target presentation in experiments 1–3. (Online version in colour.)
In summary, responses were faster to targets appearing on the face that had looked at the object to which the participant had looked. This is in line with our prediction and is the first evidence that faces that follow our gaze to an object capture attention. Indeed, in this experiment, attention shifted in the opposite direction to that which would be predicted by ‘gaze cueing’, which is somewhat surprising given how powerful gaze following can be. Could our data in fact reflect gaze cueing after the engagement of inhibitory mechanisms of attention, namely inhibition of return (IOR) [38]? This is unlikely as IOR is extremely difficult to observe in gaze cueing paradigms, emerging only at very long SOAs exceeding 2000 ms [39,40]. Our effects emerge at 100 and 400 ms SOAs—time intervals at which it is not straightforward to elicit IOR even with sudden onset cues [39–41]. Facilitation of attention towards the location of a person who has followed one's gaze is the parsimonious explanation for our observation. This therefore shows for the first time that attention orients towards a face that has looked at the same object to which an initiator of joint attention has looked: the gaze leading effect.
3. Experiment 2
It is necessary to replicate this novel finding and to validate our paradigm. In experiment 2, we simplified the display by removing the peripheral faces, providing only two target locations (the innermost locations). The outermost target locations have been informative in experiment 1, being consistent with—but not critical to the evaluation of—our hypothesis.
(a). Method
Thirty-two adults (mean age = 19.6 years, s.d. = 1.5 years; five men) took part. Owing to technical difficulties three participants completed only three of the four blocks. The stimulus display was similar to experiment 1, except that only two faces were displayed (in the innermost positions). There were now only two possible target locations, and thus half as many trials (four blocks of 32 trials) in a 2 (target location) × 2 (SOA) repeated measures design. Experimenter S.G.E. tested all participants.
(b). Results and discussion
(i). Accuracy
A 2 (target location: joint attention, non-joint attention) × 2 (SOA: 100, 400 ms) repeated-measures ANOVA was carried out on the accuracy rates of participants (97.2% of trials, s.d. = 2.69%). The effect of target location was non-significant (F1,31 = 3.81, p = 0.060, 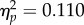 ); the trend is in the same direction as the RT effect (described below). The main effect of SOA was significant (F1,31 = 12.0, p = 0.002,
); the trend is in the same direction as the RT effect (described below). The main effect of SOA was significant (F1,31 = 12.0, p = 0.002, 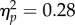 ). The interaction was not significant (F1,31 < 1).
). The interaction was not significant (F1,31 < 1).
(ii). Reaction times
A total of 1.5% of trials were discarded as outliers. A 2 (target location: joint attention, non-joint attention) × 2 (SOA: 100, 400 ms) repeated-measures ANOVA revealed a significant main effect of target location (F1,31 = 8.03, p = 0.008, 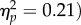 due to faster responses at the joint attention (616 ms) versus non-joint attention (630 ms) location. There was a significant main effect of SOA (F1,31 = 59.0, p < 0.001,
due to faster responses at the joint attention (616 ms) versus non-joint attention (630 ms) location. There was a significant main effect of SOA (F1,31 = 59.0, p < 0.001, 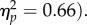 The interaction was not significant (F1,31 < 1). The effect of target location was not significant at the 100 ms SOA (t31 = −1.66, p = 0.11, dz = 0.29) and was significant at the 400 ms SOA (t31 = −2.10, p = 0.044, dz = 0.37). Therefore, we replicated the gaze leading effect, again showing that attention shifts preferentially towards the face of an individual that has followed the participant's gaze.
The interaction was not significant (F1,31 < 1). The effect of target location was not significant at the 100 ms SOA (t31 = −1.66, p = 0.11, dz = 0.29) and was significant at the 400 ms SOA (t31 = −2.10, p = 0.044, dz = 0.37). Therefore, we replicated the gaze leading effect, again showing that attention shifts preferentially towards the face of an individual that has followed the participant's gaze.
4. Experiment 3
In experiments 1 and 2, only the joint attention face looks towards an object. Thus, it may be plausible that instead of this phenomenon being a ‘gaze leading effect’, related to shared attention, it could be that observing a face look at an object more strongly captures attention than a face that looks away from an object. Therefore in experiment 3 we replicated experiment 2, adding copies of the centrally presented object to the far left and right of the faces (figure 2). This meant that both faces would always look in a common direction and at an identical meaningful object. However, only one of the looked-at objects would be a joint attention referent. We predicted that the gaze leading effect would again emerge under these conditions.
(a). Method
Thirty-two adults took part (mean age = 19.8 years, s.d. = 1.5 years; 11 males). Owing to technical difficulties two participants completed only three of the four blocks. The stimulus display was identical to that of experiment 2 except that the same object image that appeared at the central location simultaneously appeared to the left and right of the faces, positioned so that each face was equidistant from the central and a peripheral object (figure 2). Experimenter S.G.E. tested all participants.
(b). Results and discussion
(i). Accuracy
Participants responded correctly on 96.17% of trials (s.d. = 3.58%). There was no main effect of target location or SOA (Fs1,31 < 1), but there was a significant interaction (F1,31 = 6.48, p = 0.016, 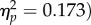 due to slightly lower accuracy for the shorter SOA at the joint attention location than at the non-joint attention location, whereas the reverse was the case at the longer SOA (table 1).
due to slightly lower accuracy for the shorter SOA at the joint attention location than at the non-joint attention location, whereas the reverse was the case at the longer SOA (table 1).
(ii). Reaction times
A total of 1.4% of trials were discarded as outliers. The main effect of target location was significant (F1,31 = 4.45, p = 0.043, 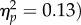 due to significantly faster responses at the joint attention (589 ms) versus non-joint attention face (599 ms) location. There was a significant main effect of SOA (F1,31 = 114, p < 0.001,
due to significantly faster responses at the joint attention (589 ms) versus non-joint attention face (599 ms) location. There was a significant main effect of SOA (F1,31 = 114, p < 0.001, 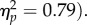 The interaction was not significant (F1,31 < 1). Like experiment 2, the effect was not reliable at the 100 ms SOA (t31 = −1.40, p = 0.17, dz = 0.25), but was at the 400 ms SOA (t31 = −2.06, p = 0.048, dz = 0.36). The gaze leading effect of faster RTs towards targets appearing on the joint attention face was again replicated. Therefore, this phenomenon is unlikely to be due solely to viewing a face look towards an object per se, but instead seems to be a result of engaging specifically in an interaction in which one's gaze is followed to a common object.
The interaction was not significant (F1,31 < 1). Like experiment 2, the effect was not reliable at the 100 ms SOA (t31 = −1.40, p = 0.17, dz = 0.25), but was at the 400 ms SOA (t31 = −2.06, p = 0.048, dz = 0.36). The gaze leading effect of faster RTs towards targets appearing on the joint attention face was again replicated. Therefore, this phenomenon is unlikely to be due solely to viewing a face look towards an object per se, but instead seems to be a result of engaging specifically in an interaction in which one's gaze is followed to a common object.
5. Relationship between the gaze leading effect and autism traits
Participants in each experiment completed the autism spectrum quotient, which is designed to assess sub-clinical autism traits [33]. Gaze leading effect magnitude was calculated for each participant (mean RT to targets appearing on ‘non-joint attention’ faces minus RT to targets on ‘joint attention’ faces). None of the individual experiments revealed a significant relationship with AQ score (experiment 1, r32 = −0.21, p = 0.25; experiment 2, r32 = −0.28, p = 0.12; experiment 3, r32 = −0.21, p = 0.26). Nevertheless, individual differences measures are less reliable with the sample sizes that we used here—principally to detect a behavioural effect at the group level (e.g. [42]). So we note, with caution, that combining the samples from experiments 1–3 revealed a significant negative relationship (r96 = −0.22, p = 0.03). This indicates that participants with more self-reported autism-like traits show weaker attentional orienting towards faces that had followed their gaze, compared with individuals with lower AQ scores. Such a relationship has been noted before between gaze cueing and the AQ [34]. This finding could prove particularly insightful regarding gaze leading, as it has been noted that social attention deficits in autism spectrum disorders may be more pronounced in initiating joint attention than gaze following [43].
6. General discussion
We have demonstrated, for the first time, that people rapidly orient their attention towards an individual who has followed their gaze and established joint attention. When participants move their eyes to a newly appeared object, they then shift their attention preferentially to a face that subsequently looks at that same object. This effect implies the existence of an attentional mechanism that prioritizes conspecifics whose overt attention we have influenced—thereby establishing a state of shared attention where both parties are aware of their common overt attention towards a referent object. This mechanism may serve a critical function in supporting social interaction and cooperation [25].
We contend that the object-based nature of joint attention is critical to the sophisticated mechanisms underpinning social orienting. That two parties are attending the same object, rather than merely orienting in the same direction, distinguishes the definition of joint attention from mere gaze following [23]. We have empirical evidence for this notion that we briefly note here. Prior to the three experiment series reported above, we conducted two preliminary experiments, similar to experiment 1. In one of these experiments (n = 33), we only displayed a fixation cross in the centre of the screen—no image of a real-world object was presented. Here, the gaze leading effect was not reliable (5 ms, p = 0.44). We propose that this is because a fixation cross does not constitute an ecologically valid ‘object’ with which one can engage in joint attention. The kitchen object stimuli used in experiments 1–3 are everyday items with an intrinsic expectation of interaction. When someone follows our gaze to an item that affords interaction we can expect that the joint attention responder will interact with that object [44,45] or make an inference about our state of mind about it [46].
It is notable that although this orienting behaviour is consistent with evolutionary [23] and developmental [29] theories of social cognition, this effect necessarily implies that to achieve a state of shared attention, one must counterintuitively orient in the opposite direction to observed gaze. Our data therefore are in direct opposition to dozens of reports on gaze cueing (see [4] for review), including some papers that presented faces outside the fovea [3,47]. So it is very surprising that our stimulus array did not produce gaze cueing. However, in another preliminary experiment (n = 18), we did demonstrate gaze cueing could emerge with our stimuli. Here, we used no referent object image but moreover we did not ask participants to make an ‘initiation’ eye movement. Here, participants oriented in the direction of observed gaze (11 ms gaze cueing effect, p = 0.016). Hence, when there is no active initiation of joint attention, the exact same stimulus layout can elicit a different social orienting response. This demonstrates that these effects are highly context-dependent in a manner that is directly predicted from theories of social attention that emphasize the importance of actively establishing joint attention with objects [24,48,49].
When the ‘gaze cueing’ effect was first examined in the laboratory [2,50,51], one question that was immediately posed was whether gaze cueing reflected the operation of specialized social mechanisms. The finding that arrow cues cause an almost identical cueing effect implies that a domain general attention mechanism could underpin both effects [28]. Does the same apply to the gaze leading effect? In a follow-up experiment identical to experiment 1 but with arrows in oval placeholders instead of faces, we found no effect of cue direction (n = 31, p = 0.80). So this suggests that, unlike gaze cueing, the gaze leading effect is an orienting response that only faces can elicit, and therefore could be a special form of social orienting. However, we make this claim with caution because the explanatory power of direct comparisons between peripherally presented gaze and arrow cues is not completely clear (but see [52,53]).
If an individual does not efficiently detect the effects they have had on their social partners, they may be less likely to continue successfully with on-going reciprocal interactions. So it is interesting to note that, across experiments 1–3, the overall strength of the gaze leading phenomenon correlated negatively with participants' self-reported autism-like traits. Therefore, a particularly important avenue for future work is to investigate whether this orienting response is present in clinical groups with impaired spontaneous social orienting (e.g. autism spectrum conditions [54] and neuropsychological patients [17,55]). If this social orienting response is particularly impaired in autism, then this could go some way to accounting for deficits in initiating joint attention behaviours found in these individuals [56].
The gaze leading effect is likely to be driven by neural substrates dedicated to gaze processing, but the data suggest that it dissociates from gaze cueing. This supports the notion that distinct cognitive and neural mechanisms are engaged when initiating joint attention, compared with responding to social gaze [9,10,14,57]. We suggest that the underlying mechanism relies on the peripheral detection of gaze, probably supported by neural structures such as the amygdala [17], with the detected gaze direction being encoded in the anterior superior temporal sulcus (aSTS), and reorienting of attention driven by the inferior parietal lobule [16,18,58]. Other systems may be involved, however. Initiating joint attention has also been specifically related to brain regions associated with representing the self [56] and reward [10,14]. Future research will benefit from exploring how attention and reward systems interact in typical and atypical social attention.
A further avenue of interest might be to explore this effect as one of social influence. Detecting that you have caused an individual to re-orient their attention to align their attention with yours may be a socially rewarding experience [7,14], akin to a social approach [59] or detecting that one has been imitated [22]. We already know that macaque [60,61] and human [62,63] social attention is influenced by affiliation, perceived dominance or status of the other individual. One could speculate the converse relationship—that being followed could promote feelings of empowerment in the ‘leader’ as they exert control over others' behaviour (even incidentally). Further, the neural correlates of joint attention initiation have also been suggested to be involved with self-monitoring [57]. Therefore, an interesting future line of enquiry would be to establish whether a feeling of agency in the gaze leader is important in these episodes, for which the time course of these interactions will be critical [48].
Many other species, particularly primates, display highly sophisticated use of the social attention cues of conspecifics and other animals (see [61,64–67]). Although many species follow gaze, skills in initiating joint attention are better developed in humans (e.g. [68,9]; see also [56]). On one hand, gaze leading may be unique to humans. This is possible because we know that shared attention is important in human language acquisition [27], making it possible that the phenomenon we report reflects an attentional mechanism that co-evolved with language. On the other hand, we also know that information transfer of heading direction, in human and animal groups, can emerge via local interactions between individuals [69–71], including human social attention [72]. It is possible that gaze leading could be an additional factor in social group interaction as information is propagated back to its original source as feedback that would influence iterative leader–follower interactions.
In conclusion, we suggest that the ‘gaze leading’ orienting response underpins the establishment of shared attention and promotes the continuation of a reciprocal social interaction. The finding may provide insights into conditions associated with deficits in social cognition, human development and comparative psychology. These data show that social orienting responses are critical not only for processing first-order social behaviour but also for interpreting the iterative effects that our own social signals have on the behaviour of others.
Ethics
Ethical approval was granted by the School of Psychology's Ethics Committee at the University of East Anglia. Written consent was obtained from each participant prior to participation.
Data accessibility
The data are available on the Dryad Digital Repository http://dx.doi.org/10.5061/dryad.8bt42.
Authors' contributions
S.G.E. and A.P.B. conceived the idea and experimental design. S.G.E. and M.D. developed and programmed the experiments. S.G.E. and L.J.S. collected the data. S.G.E. analysed the data. S.G.E., L.J.S. and A.P.B. interpreted the results. S.G.E. and A.P.B. wrote the paper. All authors made critical revisions to drafts and approved the final version for submission.
Competing interests
We have no competing interests.
Funding
This work was supported by a University of East Anglia postgraduate studentship to S.G.E., an Experimental Psychology Society Undergraduate Research Bursary to L.J.S., and a co-financed MIUR (Italian Ministry of Education, University and Research) and University of Padova postgraduate studentship to M.D.
References
- 1.Moore C, Dunham P (eds). 1995. Joint attention: its origins and role in development. Hillsdale, NJ: Erlbaum. [Google Scholar]
- 2.Driver J, Davis G, Ricciardelli P, Kidd P, Maxwell E, Baron-Cohen S. 1999. Gaze perception triggers reflexive visuospatial orienting. Visual Cogn. 6, 509–540. ( 10.1080/135062899394920) [DOI] [Google Scholar]
- 3.Friesen CK, Kingstone A. 2003. Abrupt onsets and gaze direction cues trigger independent reflexive attentional effects. Cognition 87, B1–B10. ( 10.1016/S0010-0277(02)00181-6) [DOI] [PubMed] [Google Scholar]
- 4.Frischen A, Bayliss AP, Tipper SP. 2007. Gaze cueing of attention: visual attention, social cognition, and individual differences. Psychol. Bull. 133, 694–724. ( 10.1037/0033-2909.133.4.694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nummenmaa L, Calder AJ. 2009. Neural mechanisms of social attention. Trends Cogn. Sci. 13, 135–143. ( 10.1016/j.tics.2008.12.006) [DOI] [PubMed] [Google Scholar]
- 6.Jenkins R, Beaver JD, Calder AJ. 2006. I thought you were looking at me: direction-specific aftereffects in gaze perception. Psychol. Sci. 17, 506–513. ( 10.1111/j.1467-9280.2006.01736.x) [DOI] [PubMed] [Google Scholar]
- 7.Schilbach L, Timmermans B, Reddy V, Costall A, Bente G, Schlicht T, Vogeley K. 2013. Towards a second-person neuroscience. Behav. Brain Sci. 36, 393–462. ( 10.1017/S0140525X12000660) [DOI] [PubMed] [Google Scholar]
- 8.Mundy P, Gomes A. 1998. Individual differences in joint attention skill development in the second year. Infant Behav. Dev. 21, 469–482. ( 10.1016/S0163-6383(98)90020-0) [DOI] [Google Scholar]
- 9.Mundy P, Newell L. 2007. Attention, joint attention, and social cognition. Curr. Dir. Psychol. Sci. 16, 269–274. ( 10.1111/j.1467-8721.2007.00518.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redcay E, Kleiner M, Saxe R. 2012. Look at this: the neural correlates of initiating and responding to bids for joint attention. Front. Hum. Neurosci. 6, 169 ( 10.3389/fnhum.2012.00169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilms M, Schilbach L, Pfeiffer U, Bente G, Fink GR, Vogeley K. 2010. It's in your eyes—using gaze-contingent stimuli to create truly interactive paradigms for social cognitive and affective neuroscience. Soc. Cogn. Affect. Neurosci. 5, 98–107. ( 10.1093/scan/nsq024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayliss AP, Murphy E, Naughtin CK, Kritikos A, Schilbach L, Becker SI. 2013. ‘Gaze leading’: initiating simulated joint attention influences eye movements and choice behavior. J. Exp. Psychol. Gen. 142, 76–92. ( 10.1037/a0029286) [DOI] [PubMed] [Google Scholar]
- 13.Gordon I, Eilbott JA, Feldman R, Pelphrey KA, Vander Wyk BC. 2013. Social, reward, and attention brain networks are involved when online bids for joint attention are met with congruent versus incongruent responses. Soc. Neurosci. 8, 544–554. ( 10.1080/17470919.2013.832374) [DOI] [PubMed] [Google Scholar]
- 14.Schilbach L, Wilms M, Eickhoff SB, Romanzetti S, Tepest R, Bente G, Shah NJ, Fink GR, Vogeley K. 2010. Minds made for sharing: initiating joint attention recruits reward-related neurocircuitry. J. Cogn. Neurosci. 22, 2702–2715. ( 10.1162/jocn.2009.21401) [DOI] [PubMed] [Google Scholar]
- 15.Kim K, Mundy P. 2012. Joint attention, social-cognition, and recognition memory in adults. Front. Hum. Neurosci. 6, 172 ( 10.3389/fnhum.2012.00172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perrett DI, Hietanen JK, Oram MW, Benson PJ, Rolls ET. 1992. Organization and functions of cells responsive to faces in the temporal cortex [and discussion]. Phil. Trans. R. Soc. Lond. B 335, 23–30. ( 10.1098/rstb.1992.0003) [DOI] [PubMed] [Google Scholar]
- 17.Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. 2005. A mechanism for impaired fear recognition after amygdala damage. Nature 433, 68–72. ( 10.1038/nature03086) [DOI] [PubMed] [Google Scholar]
- 18.Calder AJ, Beaver JD, Winston JS, Dolan RJ, Jenkins R, Eger E, Henson RN. 2007. Separate coding of different gaze directions in the superior temporal sulcus and inferior parietal lobule. Curr. Biol. 17, 20–25. ( 10.1016/j.cub.2006.10.052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meltzoff AN, Decety J. 2003. What imitation tells us about social cognition: a rapprochement between developmental psychology and cognitive neuroscience. Phil. Trans. R. Soc. Lond. B 358, 491–500. ( 10.1098/rstb.2002.1261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haun DB, Call J. 2008. Imitation recognition in great apes. Curr. Biol. 18, R288–R290. ( 10.1016/j.cub.2008.02.031) [DOI] [PubMed] [Google Scholar]
- 21.Paukner A, Anderson JR, Borelli E, Visalberghi E, Ferrari PF. 2005. Macaques (Macaca nemestrina) recognize when they are being imitated. Biol. Lett. 1, 219–222. ( 10.1098/rsbl.2004.0291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nadel J. 2002. Imitation and imitation recognition: functional use in preverbal infants and nonverbal children with autism. In The imitative mind: development, evolution, and brain bases (eds Meltzoff AN, Prinz W), pp. 42–62. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 23.Emery NJ. 2000. The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci. Biobehav. Rev. 24, 581–604. ( 10.1016/S0149-7634(00)00025-7) [DOI] [PubMed] [Google Scholar]
- 24.Tomasello M, Carpenter M. 2007. Shared intentionality. Dev. Sci. 10, 121–125. ( 10.1111/j.1467-7687.2007.00573.x) [DOI] [PubMed] [Google Scholar]
- 25.Moll H, Tomasello M. 2007. Cooperation and human cognition: the Vygotskian intelligence hypothesis. Phil. Trans. R. Soc. B 362, 639–648. ( 10.1098/rstb.2006.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saxe R. 2006. Uniquely human social cognition. Curr. Opin. Neurobiol. 16, 235–239. ( 10.1016/j.conb.2006.03.001) [DOI] [PubMed] [Google Scholar]
- 27.Baldwin DA. 1995. Understanding the link between joint attention and language. In Joint attention: its origins and role in development (eds Moore C, Dunham PJ), pp. 131–158. New York, NY: Psychology Press. [Google Scholar]
- 28.Böckler A, van der Wel RPRD, Welsh TN. 2014. Catching eyes: effects of social and nonsocial cues on attention capture. Psychol. Sci. 25, 720–727. ( 10.1177/0956797613516147) [DOI] [PubMed] [Google Scholar]
- 29.Mundy P, Block J, Delgado C, Pomares Y, Van Hecke AV, Parlade MV. 2007. Individual differences and the development of joint attention in infancy. Child Dev. 78, 938–954. ( 10.1111/j.1467-8624.2007.01042.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simmons JP, Nelson LD, Simonsohn U. 2012. A 21 word solution. See http://ssrn.com/abstract=2160588.
- 31.Bayliss AP, Bartlett J, Naughtin CK, Kritikos A. 2011. A direct link between gaze perception and social attention. J. Exp. Psychol. Hum. Percept. Perform. 37, 634–644. ( 10.1037/a0020559) [DOI] [PubMed] [Google Scholar]
- 32.Bayliss AP, Paul MA, Cannon PR, Tipper SP. 2006. Gaze cuing and affective judgments of objects: I like what you look at. Psychon. Bull. Rev. 13, 1061–1066. ( 10.3758/BF03213926) [DOI] [PubMed] [Google Scholar]
- 33.Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. 2001. The autism-spectrum quotient (AQ): Evidence from asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disorders 31, 5–17. ( 10.1023/A:1005653411471) [DOI] [PubMed] [Google Scholar]
- 34.Bayliss AP, Tipper SP. 2005. Gaze and arrow cueing of attention reveals individual differences along the autism-spectrum as a function of target context. Br. J. Psychol. 96, 95–114. ( 10.1348/000712604X15626) [DOI] [PubMed] [Google Scholar]
- 35.Bayliss AP, di Pellegrino G, Tipper SP. 2005. Sex differences in eye gaze and symbolic cueing of attention. Q. J. Exp. Psychol. 58A, 631–650. ( 10.1080/02724980443000124) [DOI] [PubMed] [Google Scholar]
- 36.Bayliss AP, Tipper SP. 2006. Predictive gaze cues and personality judgments: should eye trust you? Psychol. Sci. 17, 514–520. ( 10.1111/j.1467-9280.2006.01737.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loftus GR, Masson MEJ. 1994. Using confidence intervals in within-subject designs. Psychon. Bull. Rev. 1, 476–490. ( 10.3758/BF03210951) [DOI] [PubMed] [Google Scholar]
- 38.Posner MI, Cohen Y. 1984. Components of visual orienting. Attent. Perform. X Control Lang. Process. 32, 531–556. [Google Scholar]
- 39.Frischen A, Tipper SP. 2004. Orienting attention via observed gaze shift evokes longer term inhibitory effects: implications for social interactions, attention, and memory. J. Exp. Psychol. Gen. 133, 516–533. ( 10.1037/0096-3445.133.4.516) [DOI] [PubMed] [Google Scholar]
- 40.Frischen A, Smilek D, Eastwood JD, Tipper SP. 2007. Inhibition of return in response to gaze cues: the roles of time course and fixation cue. Visual Cogn. 15, 881–895. ( 10.1080/13506280601112493) [DOI] [Google Scholar]
- 41.Taylor TL, Klein RM. 1998. On the causes and effects of inhibition of return. Psychon. Bull. Rev. 5, 625–643. ( 10.3758/BF03208839) [DOI] [Google Scholar]
- 42.VanVoorhis CRW, Morgan BL. 2007. Understanding power and rules of thumb for determining sample sizes. Tutorials Quant. Methods Psychol. 3, 43–50. [Google Scholar]
- 43.Mundy P. 2003. Annotation: The neural basis of social impairments in autism: the role of the dorsal medial-frontal cortex and anterior cingulate system. J. Child Psychol. Psychiatry 44, 793–809. ( 10.1111/1469-7610.00165) [DOI] [PubMed] [Google Scholar]
- 44.Gibson JJ. 2015. The ecological approach to visual perception. New York, NY: Psychology Press. [Google Scholar]
- 45.Makris S, Hadar AA, Yarrow K. 2013. Are object affordances fully automatic? A case of covert attention. Behav. Neurosci. 127, 797 ( 10.1037/a0033946) [DOI] [PubMed] [Google Scholar]
- 46.Baron-Cohen S, Campbell R, Karmiloff-Smith A, Grant J, Walker J. 1995. Are children with autism blind to the mentalistic significance of the eyes? Br. J. Dev. Psychol. 13, 379–398. ( 10.1111/j.2044-835X.1995.tb00687.x) [DOI] [Google Scholar]
- 47.Kingstone A, Friesen CK, Gazzaniga MS. 2000. Reflexive joint attention depends on lateralized cortical connections. Psychol. Sci. 11, 159–166. ( 10.1111/1467-9280.00232) [DOI] [PubMed] [Google Scholar]
- 48.Pfeiffer UJ, Schilbach L, Jording M, Timmermans B, Bente G, Vogeley K. 2012. Eyes on the mind: investigating the influence of gaze dynamics on the perception of others in real-time social interaction. Front. Psychol. 3, 537 ( 10.3389/fpsyg.2012.00537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lobmaier JS, Fischer MH, Schwaninger A. 2006. Objects capture perceived gaze direction. Exp. Psychol. 53, 117–122. ( 10.1027/1618-3169.53.2.117) [DOI] [PubMed] [Google Scholar]
- 50.Friesen CK, Kingstone A. 1998. The eyes have it! Reflexive orienting is triggered by nonpredictive gaze. Psychon. Bull. Rev. 5, 490–495. ( 10.3758/BF03208827) [DOI] [Google Scholar]
- 51.Hood BM, Willen JD, Driver J. 1998. Adult's eyes trigger shifts of visual attention in human infants. Psychol. Sci. 9, 131–134. ( 10.1111/1467-9280.00024) [DOI] [Google Scholar]
- 52.Ristic J, Friesen CK, Kingstone A. 2002. Are eyes special? It depends on how you look at it. Psychon. Bull. Rev. 9, 507–513. ( 10.3758/BF03196306) [DOI] [PubMed] [Google Scholar]
- 53.Tipples J. 2002. Eye gaze is not unique: automatic orienting in response to uninformative arrows. Psychon. Bull. Rev. 9, 314–318. ( 10.3758/BF03196287) [DOI] [PubMed] [Google Scholar]
- 54.Jones W, Klin A. 2013. Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature 504, 427–431. ( 10.1038/nature12715) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolf RC, Philippi CL, Motzkin JC, Baskaya MK, Koenigs M. 2014. Ventromedial prefrontal cortex mediates visual attention during facial emotion recognition. Brain 137, 1772–1780. ( 10.1093/brain/awu063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tomasello M, Carpenter M, Call J, Behne T, Moll H. 2005. Understanding and sharing intentions: the origins of cultural cognition. Behav. Brain Sci. 28, 675–691. [DOI] [PubMed] [Google Scholar]
- 57.Redcay E, Saxe R. 2013. Do you see what I see? The neural bases of joint attention. In Agency and joint attention (eds Metcalfe JM, Terrace HS), pp. 216–237. Oxford, UK: Oxford University Press. [Google Scholar]
- 58.Perrett DI, Smith PA, Potter DD, Mistlin AJ, Head AS, Milner AD, Jeeves MA. 1984. Neurones responsive to faces in the temporal cortex: studies of functional organization, sensitivity to identity and relation to perception. Hum. Neurobiol. 3, 197–208. [PubMed] [Google Scholar]
- 59.Hietanen JK, Leppänen JM, Peltola MJ, Linna-aho K, Ruuhiala HJ. 2008. Seeing direct and averted gaze activates the approach–avoidance motivational brain systems. Neuropsychologia 46, 2423–2430. ( 10.1016/j.neuropsychologia.2008.02.029) [DOI] [PubMed] [Google Scholar]
- 60.Shepherd SV, Deaner RO, Platt ML. 2006. Social status gates social attention in monkeys. Curr. Biol. 18, R119–R120. ( 10.1016/j.cub.2006.02.013) [DOI] [PubMed] [Google Scholar]
- 61.Micheletta J, Waller BM. 2012. Friendship affects gaze following in a tolerant species of macaque, Macaca nigra. Anim. Behav. 83, 459–467. ( 10.1016/j.anbehav.2011.11.018) [DOI] [Google Scholar]
- 62.Jones BC, DeBruine LM, Main JC, Little AC, Welling LLM, Feinberg DR, Tiddeman BP. 2010. Facial cues of dominance modulate the short-term gaze-cueing effect in human observers. Proc. R. Soc. B 277, 617–624. ( 10.1098/rspb.2009.1575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dalmaso M, Pavan G, Castelli L, Galfano G. 2012. Social status gates social attention in humans. Biol. Lett. 8, 450–452. ( 10.1098/rsbl.2011.0881) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tomasello M, Call J. 1997. Primate cognition. Oxford, UK: Oxford University Press. [Google Scholar]
- 65.Kano F, Call J. 2014. Cross-species variation in gaze following and conspecific preference among great apes, human infants and adults. Anim. Behav. 91, 137–150. ( 10.1016/j.anbehav.2014.03.011) [DOI] [Google Scholar]
- 66.Smet AF, Byrne RW. 2014. African elephants (Loxodonta africana) recognize visual attention from face and body orientation. Biol. Lett. 10, 20140428 ( 10.1098/rsbl.2014.0428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Téglás E, Gergely A, Kupán K, Miklósi Á, Topál J. 2012. Dogs’ gaze following is tuned to human communicative signals. Curr. Biol. 22, 209–212. ( 10.1016/j.cub.2011.12.018) [DOI] [PubMed] [Google Scholar]
- 68.Tomasello M, Carpenter M. 2005. The emergence of social cognition in three young chimpanzees. Monogr. Soc. Res. Child Dev. 70, 132 ( 10.1111/j.1540-5834.2005.00332.x) [DOI] [PubMed] [Google Scholar]
- 69.Couzin ID, Krause J, Franks NR, Levin SA. 2005. Effective leadership and decision-making in animal groups on the move. Nature 433, 513–516. ( 10.1038/nature03236) [DOI] [PubMed] [Google Scholar]
- 70.Couzin ID. 2009. Collective cognition in animal groups. Trends Cogn. Sci. 13, 36–43. ( 10.1016/j.tics.2008.10.002) [DOI] [PubMed] [Google Scholar]
- 71.Dyer JR, Johansson A, Helbing D, Couzin ID, Krause J. 2009. Leadership, consensus decision making and collective behaviour in humans. Phil. Trans. R. Soc. B 364, 781–789. ( 10.1098/rstb.2008.0233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gallup AC, Hale JJ, Sumpter DJ, Garnier S, Kacelnik A, Krebs JR, Couzin ID. 2012. Visual attention and the acquisition of information in human crowds. Proc. Natl Acad. Sci. USA 109, 7245–7250. ( 10.1073/pnas.1116141109) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available on the Dryad Digital Repository http://dx.doi.org/10.5061/dryad.8bt42.