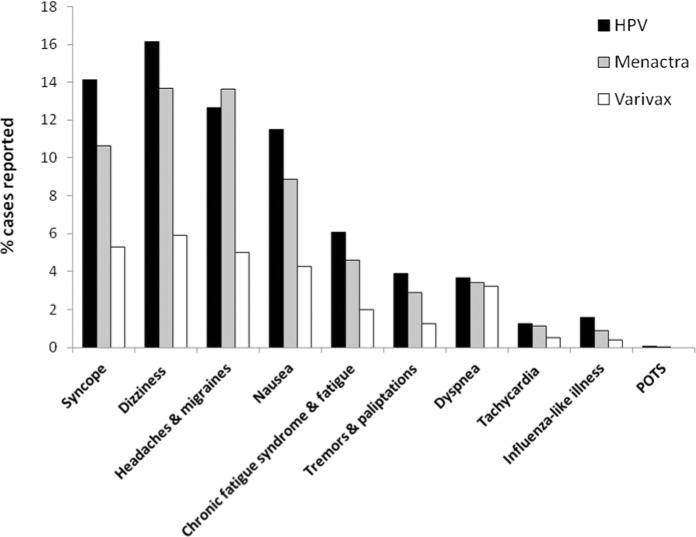
Figure 1.
Number of adverse event reports related to POTS/CFS following HPV, Menactra meningococcal polysaccharide diphtheria toxoid conjugate, and Varivax Varicella vaccines in the US Vaccine Adverse Event Reporting System (VAERS) as of February 13, 2013.
The VAERS database33 was searched using the following criteria: (1) Symptoms: syncope (general, exertional, postural); headaches (including migraines); nausea; chronic fatigue syndrome (including general fatigue); tremors and palpitations; dyspnea (general, exertional, at rest); tachycardia (including tachyarrhythmia, tachycardia paroxysomal, heart rate abnormal, heart rate increased, heart rate irregular); influenza-like illness (including viremia, viral infection); POTS; (2) Vaccine products: HPV, HPV2 (human papilloma virus bivalent), HPV4 (human papilloma virus types 6, 11, 16,1 8); MNQ (Meningococcal vaccine Menactra); Varcel (Varivax-Varicella virus live); (3) Gender (all genders); (4) Age (6 to 29 years; target age group for HPV, Menactra and Varivax vaccines); (5) Territory (the United States); (6) Date vaccinated (2007-2013; HPV vaccine postlicensure period).34 Adverse events related to a particular symptom are reported as percentages of the total number of events reported for the particular vaccine (ie, 14% syncope refers to the 2354 reports of syncope out of a total of 16 644 adverse events associated with the HPV vaccine; the total number of adverse events reported for Varivax and Menactra was 9136 and 8790, respectively).