Abstract
In songbirds, such as canaries (Serinus canaria), the song control circuit has been shown to undergo a remarkable change in morphology in response to exogenous testosterone (T). It is also well established that HVC, a telencephalic nucleus involved in song production, is significantly larger in males than in females. T regulates seasonal changes in HVC volume in males and exposure to exogenous T in adult females increases HVC volume and singing activity such that their song becomes more male-like in frequency and structure. However, whether there are sex differences in the ability of T to modulate changes in the song system and song behavior has not been investigated in canaries. In this study, we compared the effects of increasing doses of T on singing and song control nuclei volumes in adult male and female American Singer canaries exposed to identical environmental conditions. Males were castrated and all birds were placed on short days (8L:16D) for 8 weeks. Males and females were implanted either with a 2, 6 or 12 mm long Silastic™ implant filled with crystalline T or an empty 12 mm implant as control. Birds were then housed individually in sound attenuated chambers. Brains were collected from six birds from each group after 1 week or 3 weeks of treatment. Testosterone was not equally effective in increasing singing activity in both males and females. Changes in song quality and occurrence rate took place after a shorter latency in males than in females however, females did undergo marked changes in a number of measures of song behavior if given sufficient time. Males responded with an increase in HVC volume at all three doses. In females, T-induced changes in HVC volume only had limited amplitude and these volumes never reached male-typical levels a suggesting that there are sex differences in the neural substrate that responds to T.
Keywords: Song, birdsong, HVC, testosterone, brain plasticity, sex differences
1. Introduction
In temperate zone songbird species such as canaries (Serinus canaria), photoperiodic changes, along with a variety of supplementary cues, regulate the timing of the breeding season and modulate the associated anatomical, physiological, and behavioral changes (e.g., Nottebohm 1981; Wingfield and Kenagy 1991; Wingfield et al., 1993; Leitner et al., 2001; Hurley et al., 2008). In spring, gonadal volume and blood concentrations of testosterone increase in parallel with increases in day length. This change in physiological state is sufficient, although not necessary (Ball, 1999), to facilitate an increase in song behavior (Schlinger 1997; Ball et al., 2003; Harding 2004). Such a change in song behavior during the breeding season is thought to serve two functions: mate acquisition and territory defense (Baker et al, 1981; Catchpole, 1980, 1982; Darwin, 1871; King and West 1977; Krebs, 1977; Kroodsma, 1976; Marler, 1956).
In many songbird species, males and females differ in the rate and quality of song as well as in the morphology of the controlling neural substrate (Nottebohm and Arnold, 1976; Arai et al., 1989; Kirn et al., 1989; Brenowitz et al., 1991; Whitfield-Rucker and Cassone, 1996; see Ball et al., 2008 for a review). Male temperate zone songbirds tend to sing songs that are longer in duration, acoustically more complex, and produced at higher rates compared to females (see Ball et al., 2008; Catchpole and Slater 2008 for reviews). In wild and domesticated canaries, Serinus canaria, this sex difference in the production of song is also observed (e.g., Poulsen, 1959; Nottebohm et al., 1986; Leitner et al, 2001 Leitner and Catchpole, 2002). However, there is wide variation in the naturally occurring behavior of female temperate zone songbirds and in the ability of exogenous administration of testosterone (T) to pharmacologically induce in females male-like patterns of brain and behavior (Riebel, 2003; Harding, 2004). Adult female canaries, that normally sing less frequent and simpler songs than males, sing with greater frequency and quality in response to T-treatment (Gahr and Garcia-Segura, 1996; Hartog et al., 2009). Likewise, the volumes of song control nuclei in T-treated adult female canaries tend to be larger than those of non-treated adult females (Nottebohm, 1980). T-treatment in adult female songbirds induces a cascade of molecular, morphological, and behavioral changes that appear to result in the recapitulation of the sensorimotor phase of song learning so that the ensuing song sounds more male-like (Nottebohm, 1980). The activation of male-like song in female canaries seems to recapitulate a developmental learning stage typically observed in males (Nottebohm et al., 1986, 1987), and is also associated with the partial masculinization of the brain of T-treated females (Nottebohm 1980).
In captive male canaries, naturally occurring changes in T concentrations are related to the development of song progressing from subsong, to plastic song and crystallized song (Nottebohm et al., 1986; 1987). These changes in song behavior are causally related to T as T-treatment in adult male gonadectomized songbirds induces a cascade of molecular, morphological, and behavioral changes similar to changes that are observed in intact photostimulated males. Moreover, in gonadally intact adult male canaries song repertoire can vary across seasons with the addition and deletion of syllable types as a function of age. These changes parallel and are partially caused by seasonal changes in T concentration (Nottebohm et al, 1978, 1986; Leitner et al. 2001).
T thus appears to have powerful effects on song behavior and the underlying brain regions mediating song behavior in male and female canaries. However, it is not known if male and female songbirds respond to T in the same way. When adult sex differences are mediated by differences in circulating steroid concentration (i.e. activational effects rather than organizational effects early in ontogeny), it is often assumed that males and females have similar capacities to respond to T. This is not necessarily the case. It is not known if the pattern of vocal development and associated changes in neural morphology is the same in T-treated male and female songbirds. This study systematically investigates the activational effects of varying doses of T-treatment in adult male and female canaries housed under the same photoperiodic conditions.
2. Methods
2.1. Experimental animals
Forty-three male and forty-three female American singer canaries ranging from 12-18 months old were obtained from a local breeder (Maryland Exotic Birds) and housed in an indoor aviary on an 8L:16D (light:dark) light cycle for at least eight weeks. Birds were kept in 49 × 95 × 51 cm cages (six birds of the same sex per cage) at Johns Hopkins University, Baltimore, MD and fed canary food and provided water ad libitum. Care and handling of all animal subjects was in accordance with guidelines published by the National Research Council (2011) and all experimental procedures were approved by the Johns Hopkins University Institutional Animal Care and Use Committee. After eight weeks on 8L:16D, females’ ovaries were regressed, as confirmed by laparotomy, and males with regressed testes were castrated under general anesthesia using isoflurane (mix of air gas and 3% isoflurane for induction and 2.5% during the surgery). The two testes were removed through a small incision in the flank, posterior to the last rib. The incision was sutured closed and lidocaine and antibiotic ointment was placed on the wound. Males were allowed to recover under warm light before being returned to their home cages. One week after castration, blood samples were taken from each bird and only male and female canaries with undetectable blood T levels were placed on study. Females in this study were not ovariectomized based on two factors. First, many studies of T-induced adult neuroplasticity have successfully used female canaries that were not ovariectomized but had photoregressed ovaries (e.g., Goldman and Nottebohm, 1983; Hartog et al., 2009). Second, there is a high vascularization of the ovary in female canaries even in photoregressed individuals. This vascularization increases mortality rates when removing this structure. There may be some concern that the short day photosensitive males experience a castration response (i.e. an increase in Gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH) due to the removal of negative feedback from steroid hormones that will not occur in the photoregressed females thus introducing a bias in the sex comparison in the responses to T. We do not think this difference significantly influences our results as work by Storey and Nicholls (reviewed in Nicholls et al., 1988) showed that males on short days have an attenuated castration response as compared to males on long days and most males received implants of T that rapidly decreases GnRH and LH release. The castrated males that received empty implants did not produce high levels of song or large song control nuclei, in agreement with the published literature suggesting that there are no known direct effects of GnRH or LH on song behavior and brain plasticity.
Males and females remained on an 8L:16D light cycle for at least eight additional weeks before they were implanted subcutaneously with one Silastic™ implant (Dow Corning, Midland, MI, USA, no. 602-175; 0.76 mm inner diameter [ID], 1.65 mm outer diameter [OD]) of either 2 mm, 6 mm, 12 mm length filled with crystalline T or a 12 mm implant left empty as a negative control. We defined the T treatments as low, medium, and high dose respectively. Implant length and diameter were selected based on previously published studies in canaries indicating that these implant sizes are sufficient to activate singing and to increase song control nuclei size to levels characteristic of gonadally-intact males (Appeltants, et al., 2003; Nottebohm, 1980; Sartor, et al., 2005).
Final numbers of males (M) and females (F) in each treatment that were analyzed for behavioral and histological variables were as follows: week 1, control: 5 M/6 F, low: 6M/5F, medium: 5M /5F and high: 5M/5F; week 3, control: 6 M/5 F, low: 5M/5F, medium: 4M /6F and high: 6M/6F.
Starting immediately after these subcutaneous implantations, all male and female canaries were individually housed in sound attenuated chambers (41cm × 48cm × 51 cm). Song behavior was recorded for thirty minutes, three times per day for either seven or twenty one days, depending on treatment group. Isolation chambers were outfitted with a microphone (BTMP8087 Mini microphone; B&H Foto and Electronics Corp, New York, NY) and camera (KPC-600 Pinhole Camera 3.6mm; B&H Foto and Electronics Corp, New York, NY) connected to computer running DVRserver (V6.33b; Mammoth Technologies, Austin, TX) designed for real-time video and audio surveillance recording. Behavioral recordings (audio and video) began immediately after lights on (0900 hrs), four hours after lights on (1300 hrs), and thirty minutes before light off (1630 hrs). All canaries remained on an 8L:16D photoperiod for the duration of the study as it was necessary to examine the effects of the steroid hormone treatment in the absence of any other cues characteristics of breeding condition.
2.2. Behavioral Quantification
Sound files (converted from audio/video file format .mp4 to audio only .wav files; the video information was not analyzed in this study) were sampled at 22050 Hz, which translates to a frequency range of 0 to 11 kHz. Since little or no singing was recorded in the tapes collected just before lights off, they were not included in the analyses that were focused exclusively on tapes collected at 0900 and 1300 hrs. No statistical difference could be detected between these two sets of tapes and data were thus collapsed across the two times of the day.
Audio files were highpass filtered with audio editing software Goldwave ™ (version 5.55) set to a threshold of 900 Hz to remove low frequency noise (e.g. the sound of the fan/air vent and hum of the light). Sound spectrograms were created for each daily recoding using Avisoft SASlab (Avisoft Bioacoustics, Berlin, Germany). Spectrogram FFT (fast Fourier transform) lengths were set to 512 with an overlap of 75% for the temporal resolution. For each spectrogram the number of songs, song bout duration (in seconds), number of calls, the total time spent singing and/or producing vocalizations (i.e. calls in addition to songs), and other acoustic features (i.e. mean Wiener entropy, Wiener entropy variance, the number of elements per song bout, vocalization energy, and mean peak amplitude) for each recording were calculated and exported into an Excel spreadsheet.
Canary song has a characteristic acoustic structure and temporal pattern that distinguishes it from calls and we used the following criterion to differentiate songs from calls in the spreadsheet (Güttinger et al., 1978; Güttinger 1985; Nottebohm and Nottebohm, 1978; Wolffgramm, 1973). We defined song as being bouts of vocalizations where the total duration was greater than 1.5 seconds of continuous notes (featuring 5 or more notes that have a peak amplitude value greater than -22dB) with inter-syllable intervals no longer than 500 milliseconds and a mean entropy value less than W = 0.550.
In addition to measures of song rate, other acoustic features of song were measured including the aforementioned Wiener entropy variance, the number of elements per song bout, vocalization energy, and mean peak amplitude. Energy is the sum (i.e. integral) of the squared amplitudes of a sound multiplied by its sampling time (Avisoft SASlab User Manual) and is a measure of the ‘loudness’ of a given vocalization. Weiner entropy was measured in Avisoft SASlab. Wiener entropy is a measure of the spectral width and uniformity of a signal. In canaries we have found that the variance of this measure collapsed across a single bout of singing is indicative of syllable diversity and syllable stereotypy (e.g. high entropy variance is associated with the inclusion of multiple syllable/phrase types; Unpublished observations). This is similar to what is observed in juvenile male zebra finch in which entropy variance increases rapidly with the onset of sensorimotor song learning, which is marked by significant vocal variability (both in note composition and spectral stability; Tchernichovski et al., 2001; Shank and Margoliash, 2009).
In vocal communication, like birdsong, loud signals exploit the sensory modality and increase the likelihood of conspecific vocalizations being discriminated from background (Todt and Naguib, 2000; Searcy and Nowicki, 2005). Song in male songbirds functions both to attract a mate and defend a territory; males can modulate the amplitude of signals to maximize discrimination from background noise or to compete with other conspecifics (Brumm and Todt, 2002, 2004). We investigated whether males and females produced loud signals and complex signals in a similar manner in response to T. We did this by correlating the loudness of song (i.e. vocalization energy) and the rate/composition of song in both sexes.
Finally, we tracked the development of individual syllables in response to T-treatment. T is required for song/syllables to crystallize: when song crystallizes, individual syllable iterations within a bout of singing and between bouts of singing become highly similar to one another (Waser and Marler, 1977; Marler and Peters, 1982; Marler, et al, 1988). To measure the rate of syllable similarity over time we isolated high amplitude vocalizations (peak amplitude > -14dB; song elements only, calls omitted) on the final day of recording, day 21, into individual .wav files. Syllables were then randomly re-sampled (maximum 100 syllables), categorized, and template sonograms were made for selected syllable iteration. High amplitude vocalizations (peak amplitude > -14dB; song elements only, calls omitted) on the days 5, 10, 15, & 20 were then isolated into individual .wav files. Binary sonogram templates from the final day were then cross-correlated with sonograms for all song elements above amplitude threshold for days 5, 10, 15, & 20 in Avisoft SASlab. The percentage of syllable correlations above an r = 0.95 correlation threshold was then tabulated for each bird for each day (i.e. days 5, 10, 15, 20).
2.3. Serum T enzyme linked immunoassay (EIA)
Blood samples were collected into microcentrifuge tubes at the end of the experiment (after seven or twenty one days of treatment with T) from the severed carotid artery of the bird immediately after rapid decapitation. Blood was centrifuged at 9,000xg for 5 minutes resulting in separation of serum from red blood cells. Serum concentrations of T were measured using an enzyme-linked immunoassay from Enzo Life Sciences (Testosterone EIA kit; cat #ADI-901-065, Plymouth Meeting, PA). The kit was validated for use with canary serum by testing for parallelism and recovery of added mass (standard biochemical validations). To test for parallelism, high and low T pools were pipetted at five different volumes in quadruplicate to ensure that the dose response curves were parallel to the standards under dilution and to confirm that T in the sample bound with the antibody with the same affinity as T in the standard curve and that no other compound in the sample binds to the antibody but with a different affinity. Recovery of exogenous testosterone verifies accurate measurement throughout the working range of the assay. To test for recovery of added mass, three standard curve points (from the middle of the curve) were added to the high and low pool to ensure that the added mass could be accurately detected, indicating that the sample was not blocking the antibodies ability to bind with the standard. The intra- and interassay coefficients of variation were 9% and 14%, respectively.
2.4. Song nuclei volume reconstruction
At the end of the treatment period (1 week or 3 weeks), birds were euthanized by rapid decapitation and brains were extracted and placed in fixative (5% acrolein). The extracted brain was fixed via slight agitation in acrolein fixative and cryoprotected in 30% sucrose. Brains were then flash frozen on dry ice and placed in the −70°C until processed for later analysis.
Brains were sectioned at 30 μm thickness using a cyrostat (Carl Zeiss) and Nissl-stained using Thionin to visualize the song control nuclei HVC, Area X, and the robust nucleus of the arcopallium (RA). Brains sections were stained with thionin, cleared in xylene (Fisher Scientific), and coverslipped with Permount (Fisher Scientific).
Brain regions of interest were digitized using a bright field light microscope (Zeiss Axioscope, Carl Zeiss, Thornwood NY) with a CCD camera connected to a desktop computer. For each image, the area of the brain region was measured using Openlab 5.0.2 (Improvision, Lexington, MA). The volume of each region was then reconstructed combining the areas of subsequent sections with the sampling interval (120 μm) using the formula for a truncated cone (see Tramontin et al., 1998) as used previously in starlings (Bernard and Ball, 1995; Bentley et al., 1999; Bernard and Ball, 1997). For each bird we used the average volume of the nuclei measured in the left and right hemispheres.
2.5. Statistical Analysis
To analyze the song rate, ratio of song to calls, and vocalization energy data we used a split-plot factorial analysis of variance (ANOVA; 4-way mixed-design with 3 between [i.e. sex, dose, & week of tissue collection] and 1 within subjects variable [i.e. day of the week]). For these repeated measures variables, significance for main effects was corrected using a Greenhouse-Geisser correction for non-sphericity. Effect size (eta squared; 2) values are reported for the main and interaction effects only.
Daily mean values were highly variable and not normally distributed for song bout length and number of individual notes so marginal mean values for the entire week (i.e. week 1 and week 3) were used to decrease variance and normalize the data. The two behavioral measures were averaged for the entire week. These data and the T concentrations in blood samples collected at the end of the experiment thus only consisted in one time point in each experimental group. Three-way analyses of variance were therefore used to analyze these data (i.e. sex, dose, & week of tissue collection). Three-way Bonferroni-corrected multivariate analysis of variance (MANOVA; independent variables included sex, dose, & week of tissue collection) was used to discern particular patterns in the data where there were multiple contiguous dependent variables such as the song control nuclei volumes (i.e. volume of HVC, Area X, and RA). All post-hoc tests were corrected for multiple comparisons using Bonferroni's correction unless otherwise noted.
Relationships (i.e. correlations) between variables were evaluated with linear regression analyses controlling for covariance of related predictors. Regressions analyses were run in the following order: males only during week 1, females only during week 1, males only during week 3, and females only during week 3. Values for the week of observation were means collapsed across the entire week. The reported values are the individual contribution of each variable to the final regression model(s). Results were considered statistically significant for α < 0.05.
3. Results
3.1. Testosterone concentration
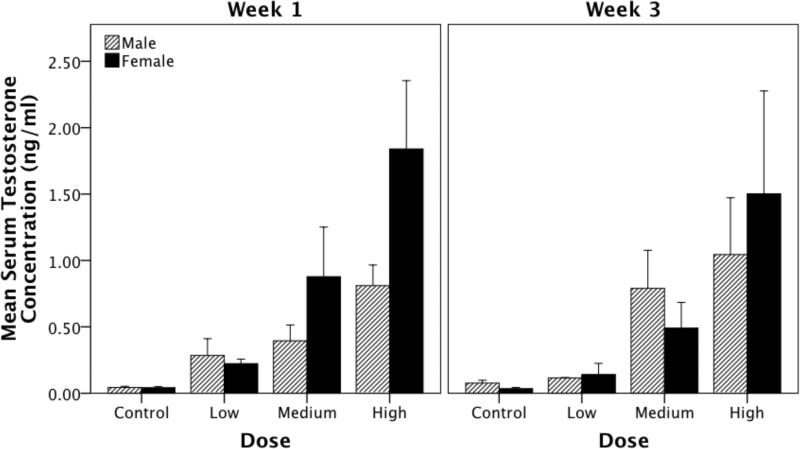
We measured the post-treatment concentrations of T in circulating serum and found a significant of effect of dose (F(3,72) = 4.885, p = 0.004, η2 = 0.205). However, there was no difference in serum T concentration between males and females nor between time of sampling (i.e. week 1 versus week 3; Fsex(1,72) = 0.130, p = 0.720; Fweek(1,72) = 0.724, p = 0.398). Likewise, there were no significant interaction effects (Fsex*dose(3,72) = 2.079, p = 0.113; Fsex*week(1,72) = 0.277, p = 0.601; Fdose*week(3,72) = 0.393, p = 0.758; Fsex*dose*week(3,72) = 1.376, p = 0.259). Though the most powerful difference between doses was between the blank control versus the high dose (i-jcontrol-high = -1134.959, p = 0.001) there was a robust linear trend showing that the size of the implant was related to T concentration in serum (p < 0.001; Figure 1).
Figure 1.
Effect of Silastic implants of various sizes filled with testosterone on serum testosterone concentrations in male and female canaries (data pooled) at one or three weeks after implantation. There was a significant effect of capsule size (i.e. dose) on serum concentrations of testosterone (T). There was, however no effect of sex (i.e. males and females had equivalent levels of T in serum) and of the duration of implantation (week).
3.2. Song rate
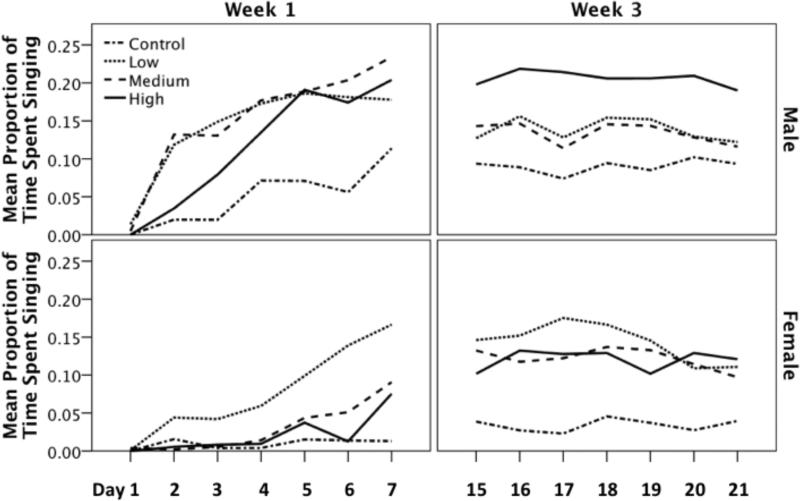
Exogenous administration of T is known to increase the singing rate of castrated male and intact female canaries and we found a significant main effect of dose on this dependent variable (F(3,68) = 3.438, p = 0.022; Figure 2). Furthermore, there was a significant main effect of sex; males sang more than females (F(1,68) = 8.792, p = 0.004). Likewise, there was a significant main effect of week; birds sang more during week 3 than week 1 (F(1,68) = 5.891, p = 0.018). There were no significant interaction effects for the between subjects variables (Table 1). Overall, birds treated with a low dose of T sang more frequently than controls (i-jcontrol-low = -0.080, p = 0.032). There was a trend in the data for birds treated with a high dose of T to sing more often than controls, however, after Bonferroni correction for multiple comparisons the trend was not significant (i-jcontrol-high = -0.070, p = 0.082).
Figure 2.
The effect of T-treatment on the rate of singing in males and females during week 1 and week 3. During week 1 of T treatment males exhibited shorter response latency to T-treatment (i.e. they sang sooner after T-treatment compared to females) and sang at a higher rate than females. However, by week three T-treated females sang at similar rates compared to males.
Table 1.
Results of the statistical analyses of the song behavior data
| Three-way ANOVAs | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percent Time Singing | Ratio of Singing to Calling | Mean Song Duration | No. of Notes Per Song | Song Energy (Loudness) | |||||||||||
| F (3,68) | p | η 2 | F (3,68) | p | η 2 | F (3,68) | p | η 2 | F (3,68) | p | η 2 | F (3,68) | p | η 2 | |
| Sex | 8.792 | 0.004** | 0.114 | 7.620 | 0.007** | 0.101 | 2.518 | 0.117 | 0.036 | 5.515 | 0.022* | 0.075 | 11.299 | < 0.001*** | 0.142 |
| Dose | 3.438 | 0.022* | 0.132 | 5.532 | 0.002** | 0.196 | 6.208 | < 0.001*** | 0.218 | 6.927 | < 0.001*** | 0.234 | 3.235 | 0.028* | 0.125 |
| Week | 5.891 | 0.018* | 0.080 | 6.954 | 0.010* | 0.093 | 4.386 | 0.040* | 0.061 | 7.268 | < 0.001*** | 0.097 | 4.324 | 0.041* | 0.060 |
| Sex * Dose | 0.434 | 0.729 | 0.019 | 0.173 | 0.914 | 0.008 | 0.276 | 0.842 | 0.012 | 0.162 | 0.922 | 0.007 | 0.899 | 0.451 | 0.038 |
| Sex * Week | 1.205 | 0.276 | 0.017 | 2.586 | 0.112 | 0.037 | 4.635 | 0.035* | 0.065 | 4.235 | 0.043* | 0.059 | 0.026 | 0.871 | 0.000 |
| Dose * Week | 0.600 | 0.617 | 0.026 | 0.442 | 0.724 | 0.019 | 0.778 | 0.510 | 0.034 | 0.582 | 0.629 | 0.025 | 1.231 | 0.305 | 0.052 |
| Sex * Dose * Week | 0.511 | 0.676 | 0.022 | 0.204 | 0.893 | 0.009 | 0.549 | 0.651 | 0.024 | 0.966 | 0.414 | 0.041 | 0.544 | 0.654 | 0.023 |
| Four-way ANOVAs | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Percent Time Singing | Ratio of Singing to Calling | Song Energy (Loudness) | |||||||
| F (6,408) | p | η 2 | F (6,408) | p | η 2 | F (6,408) | p | η 2 | |
| Day | 23.980 | < 0.001*** | 0.261 | 29.787 | < 0.001*** | 0.305 | 15.172 | < 0.001*** | 0.182 |
| Day * Sex | 5.761 | < 0.001*** | 0.078 | 1.945 | 0.104 | 0.028 | 6.868 | < 0.001*** | 0.092 |
| Day * Dose | 1.150 | 0.327 | 0.048 | 0.948 | 0.499 | 0.040 | 1.559 | 0.108 | 0.064 |
| Day *Week | 31.807 | < 0.001*** | 0.319 | 24.277 | < 0.001*** | 0.263 | 10.108 | < 0.001*** | 0.129 |
| Day * Sex* Dose | 0.924 | 0.511 | 0.039 | 1.513 | 0.120 | 0.063 | 1.349 | 0.195 | 0.056 |
| Day * Sex * Week | 5.589 | < 0.001*** | 0.076 | 4.095 | 0.003** | 0.057 | 7.312 | < 0.001*** | 0.097 |
| Day * Dose * Week | 2.263 | 0.016* | 0.091 | 1.544 | 0.109 | 0.064 | 1.157 | 0.316 | 0.049 |
| Day * Sex * Dose * Week | 1.741 | 0.073 | 0.071 | 0.622 | 0.821 | 0.027 | 1.849 | 0.044* | 0.075 |
There was a significant main effect of the repeated measure, i.e. the day of observation (F(6,408) = 23.980, p < 0.001). As expected, however, there was a significant interaction between the day of observation and week of assessment (F(6,408) = 31.807, p < 0.001). There was also a significant interaction between the day of observation and sex (F(6,408) = 5.761, p < 0.001) and a significant three way interaction between sex, week of assessment, and day of observation (F(6,408) = 5.589, p < 0.001). All other interactions of the between and within subjects variables on singing rate were not significant (see table 1).
Post-hoc analyses showed that, though during week 1 there was a significant effect of day on singing rate (F(6,246) = 31.728, p < 0.001), during week 3 this effect was only a non-significant trend (F(6,246) = 2.077, p = 0.089; Figure 2). A Bonferonni corrected MANOVA for days 1 through 7 (i.e. week 1 of treatment) revealed that males sang more than females on days 2 through 7 of T-treatment (Table 2). In contrast, a separate Bonferonni corrected MANOVA showed that males and females did not differ on any day of treatment in week 3 (Table 2).
Table 2.
Interaction of Sex (Male vs. Female), Day, and Week of Observation Post hoc analyzes of the sex differences day by day
| Percent Time Singing | Energy (Loudness) | ||||
|---|---|---|---|---|---|
| F (1,34) | p | F (1,40) | p | ||
| Week 1 | Day 1 | 0.776 | 0.385 | 0.273 | 0.604 |
| Day 2 | 9.504 | 0.004** | 1.468 | 0.233 | |
| Day 3 | 20.089 | < 0.001*** | 9.973 | 0.003** | |
| Day 4 | 25.499 | < 0.001*** | 11.127 | 0.002** | |
| Day 5 | 13.195 | < 0.001*** | 9.391 | 0.004** | |
| Day 6 | 15.257 | < 0.001 *** | 11.575 | 0.002** | |
| Day 7 | 10.393 | 0.003** | 16.619 | < 0.001*** | |
| Week 3 | Day 15 | 0.878 | 0.355 | 3.84 | 0.057† |
| Day 16 | 1.535 | 0.224 | 3.24 | 0.079† | |
| Day 17 | 0.340 | 0.564 | 1.385 | 0.246 | |
| Day 18 | 0.605 | 0.442 | 2.841 | 0.100 | |
| Day 19 | 1.378 | 0.249 | 1.553 | 0.220 | |
| Day 20 | 1.925 | 0.174 | 3.552 | 0.067† | |
| Day 21 | 1.340 | 0.255 | 2.264 | 0.140 | |
3.3. Ratio of songs versus calls
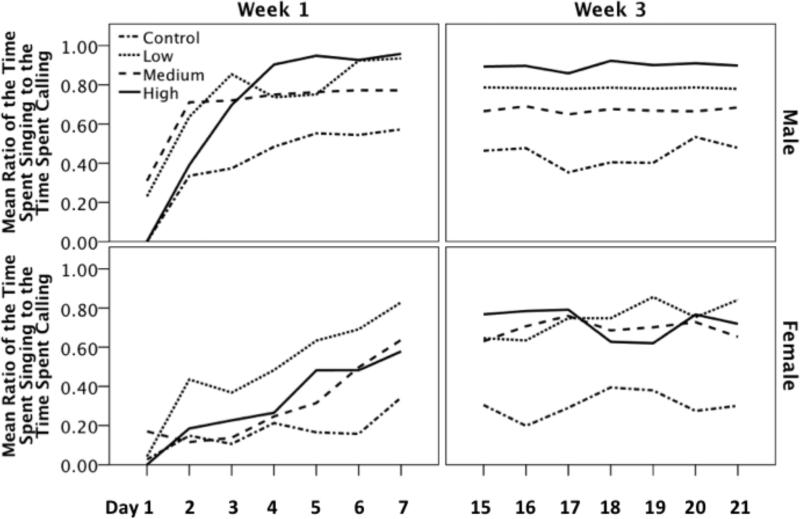
Over the recording period we measured and compared the rate of singing to the rate of calling. Analyses of this ratio revealed a significant main effect of dose (F(3,68) = 5.532, p = 0.002; Figure 3). Likewise there was a significant main effect of sex; males spent a greater proportion of time singing versus calling compared to females (F(1,68) = 7.620, p = 0.007). Furthermore, there was an effect of week; birds spent a greater proportion of time singing versus calling in week 3 compared to week 1 (F(1,68) = 6.954, p = 0.010). There were no significant interactions of the between subjects variables (Table 1). At low and high doses T-treated birds spent a greater proportion of time singing versus calling compared to controls (i-jcontrol-low = -0.361, p = 0.003; i-jcontrol-high = -0.331, p = 0.007). There was also a trend for medium dose birds to spend a greater proportion of time singing versus calling, however, after a Bonferonni correction for multiple comparisons the finding was not statistically significant (i-jcontrol-medium = 0.261, p = 0.061).
Figure 3.
The effect of T-treatment on the ratio of time spent singing to the time spent calling in males and females during week 1 and week 3 of treatment. T-treatment increased general vocalizing of all birds; however, during the first week females spent a lower proportion of that time singing versus calling compared to males. However, by week three the ratio of song to call was similar in males and females.
There was a significant main effect of the day of observation on the ratio of song to calls (F(6,408) = 29.787, p < 0.001). However, there was a significant interaction between the week of assessment and the day of observation (F(6,408) = 24.277, p < 0.001). Likewise, there was a significant interaction between sex, week of assessment, and day of observation (F(6,408) = 4.095, p = 0.003). All other interactions of the between and within subjects variables on the ratio of song to call were not significant (Table 1). During week 1 of T-treatment females spent a greater proportion of time calling versus singing as compared to males but did not differ from males in the ratio of song to calls during week 3 (Figure 3; Fweek1(1,34) = 15.171, p < 0.001; Fweek3 (1,40) = 0.259, p = 0.613). Furthermore, though there was a main effect of day of observation during week 1 of treatment (F(6,204) = 38.122, p < 0.001), the effect was not significant by week 3 (F(6,204) = 0.328, p = 0.922).
3.4. Mean song bout length
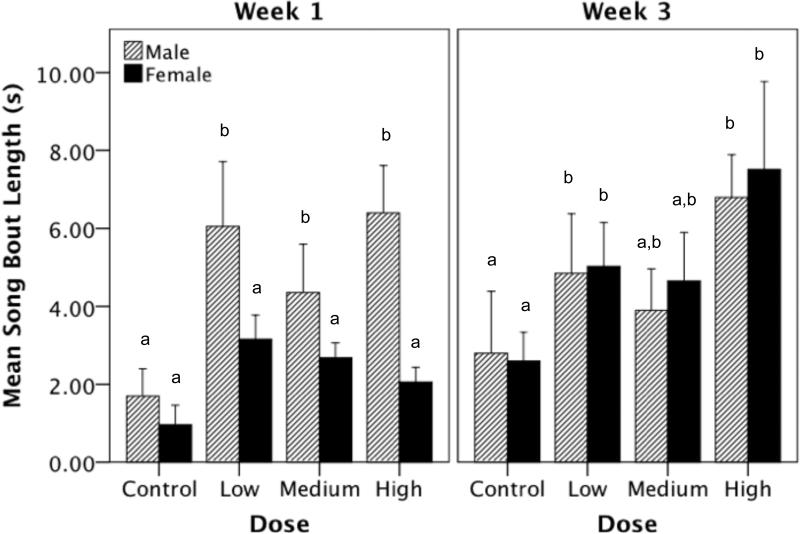
There was a significant main effect of dose on the mean song bout length (in seconds) averaged across all days of a given week (Figure 4; F(3,67) = 6.208, p < 0.001). In addition, there was a significant main effect of week; birds that sang during week 3 of observation sang longer songs than birds that sang in week 1 (F(1,67) = 4.386, p = 0.040). Though there was no main effect of sex on song bout length (F(1,67) = 2.518, p = 0.117), there was a significant interaction between sex and week of observation (F(1,67) = 4.635, p = 0.035). All other interaction effects were not significant (Table 1). Post-hoc tests revealed that the greatest dose differences observed were between the high dose versus control birds (i-jcontrol-high = -3.777, p < 0.001) and low dose versus control birds (i-jcontrol-low = -2.915, p = 0.011). Further analyses revealed that during week 1 of T-treatment, males sang longer songs than females (F(1,33) = 11.254, p = 0.002), however, this difference was not present during week 3 (F(1,34) = 0.118, p = 0.734).
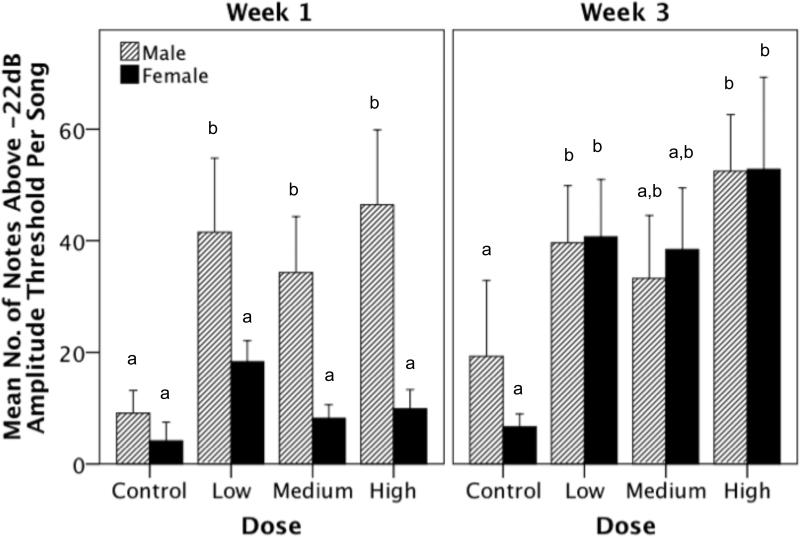
Figure 4.
The effect of T-treatment on the song bout length (mean of the entire week) in males and females during week 1 and week 3. During the first week of T-treatment males sang longer songs compared to females, however, by week three male and female T-treated birds did not differ in song bout length. Bars with a same letter are not significantly different; bars with a different letter are different for p≤0.05 in the post-hoc tests. Bars marked with an ‘a’ are significantly lower than bars marked with ‘b’. Likewise, bars marked with ‘a,b’ are not significantly different from bars marked ‘a’ or ‘b’.
3.5. Number of individual notes
As with song bout length, for the mean number of individual notes above amplitude threshold averaged across the entire week, there was a significant main effect of dose (Figure 5; F(3,68) = 6.927, p < 0.001). Furthermore, there was a significant main effect of week; birds sang more notes above threshold in week 3 of observation compared to week 1 (F(1,68) = 7.268, p = 0.009). In addition, there was a significant main effect of sex on the number of notes above threshold (i.e. males > females; F(1,68) = 5.515, p = 0.022), and importantly there was also a significant interaction between sex and week of observation (F(1,68) = 4.235, p = 0.043). All other interaction effects were not significant (Table 1). Post-hoc analyses revealed that the greatest dose difference was between the high dose versus control birds (i-jcontrol-high = -31.002, p < 0.001) and low dose versus control birds (i-jcontrol-low = -25.365, p = 0.004). There was a trend for the medium dose birds versus controls, however, after Bonferonni correction for multiple comparison the difference was not significant (i-jcontrol-medium = -18.832, p = 0.069). Furthermore, during week 1 of T-treatment, males sang more notes above amplitude threshold compared to females (F(1,33) = 14.980, p < 0.001), however, during week 3 of treatment this difference between males and females was no longer observed (F(1,34) = 0.031, p = 0.861).
Figure 5.
The effect of T-treatment on the mean number of notes per song (collapsed across the entire week) in males and females during week 1 and week 3. During week 1, male T-treated birds had more individual notes above amplitude threshold for acoustic measurement compared to females. This difference was not observed during week 3. Bars with a same letter are not significantly different; bars with a different letter are different for p≤0.05 in the post-hoc tests. Bars marked with an ‘a’ are significantly lower than bars marked with ‘b’. Likewise, bars marked with ‘a,b’ are not significantly different from bars marked ‘a’ or ‘b’.
3.6. Mean vocalization energy
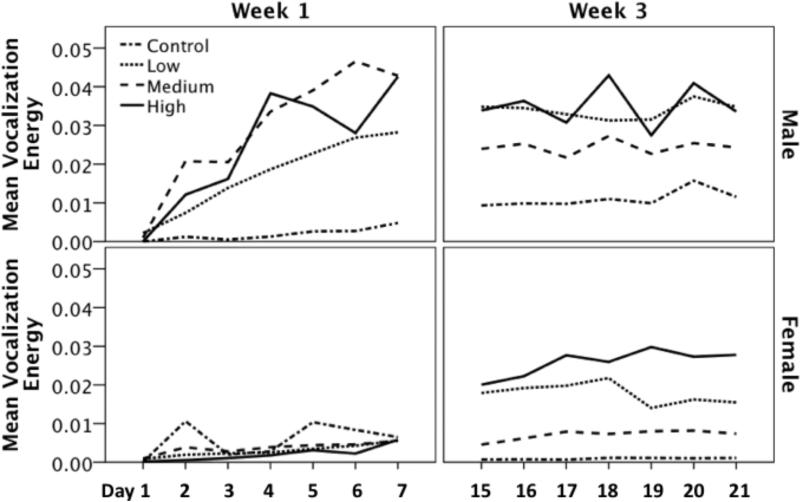
To further characterize the singing behavior of these birds, we also measured the mean vocalization energy; as previously stated, energy is a measure of the ‘loudness’ of a given vocalization (Figure 6). There was a significant main effect of dose on the mean vocalization energy (F(3,68) = 3.235, p = 0.028). In addition, there was a significant main effect of week; songs in week 3 of T-treatment were louder than songs in week 1 of T-treatment (F(1,68) = 4.324, p = 0.041). Furthermore, there was a significant main effect of sex; males tended to sing louder songs compared to females (F(1,68) = 11.299, p = 0.001). There were no significant interaction effects for the between subjects variables (Table 1). Post-hoc tests showed that overall, birds treated with a high dose of T sang louder songs compared to controls (i-jcontrol-high = -0.169, p = 0.027).
Figure 6.
The effect of T-treatment on the mean vocalization energy in males and females during week 1 and week 3. Vocalization energy is the summed (integral) amplitude peaks of a given signal. During week 1of T-treatment males sang much louder (i.e. greater energy) song compared to females. This difference was not observed during 3 three of treatment.
We also found a significant main effect of day of observation within the week, i.e. the repeated measure variable (F(6,408) = 15.172, p < 0.001). In addition, there was a significant interaction between the day of observation and week of assessment (F(6,408) = 10.108, p < 0.001). Furthermore, there was a significant interaction effect of day of observation and sex (F(6,408) = 6.868, p < 0.001) and a significant three way interaction between sex, week, and day of observation (F(6,408) = 7.312, p < 0.001). There was also a significant four way interaction between day of observation, sex, dose, and week of assessment (F(18,408) = 1.849, p = 0.044; Figure 6). All other interaction effects of the within and between subjects variables were not significant (Table 1).
Post-hoc analyses demonstrated that there was a significant effect of day of observation on vocalization energy during week 1 (F(6,246) = 13.496, p < 0.001), but not during week 3 (F(6,246) = 1.898, p = 0.116). Furthermore, a Bonferonni corrected MANOVA for days 1 through 7 (i.e. week 1 of treatment) revealed that males sang louder songs than females on days 3 through 7 of T-treatment (Table 2). A separate Bonferonni corrected MANOVA showed that males and females did not differ on any day of treatment in week 3; however, there were non-significant trends on days 15,16, and 20 (Table 2).
3.7. Correlation of song rate/composition and vocalization energy
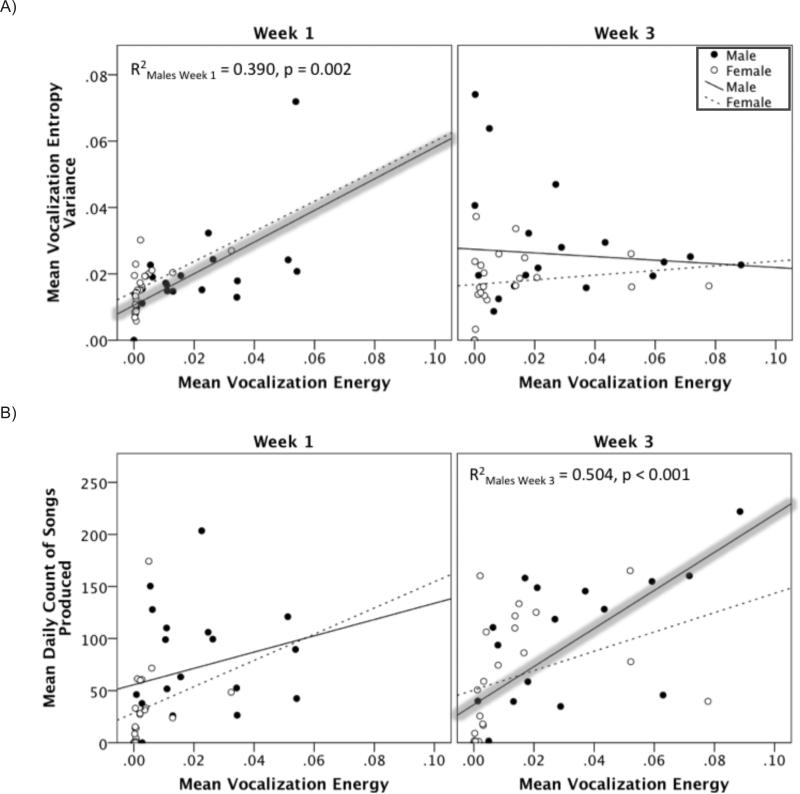
Multiple linear regression analyses revealed significant predictive relationships between the acoustic structure of song and rate of song production with vocalization energy. In particular, during week 1 of T-treatment males who tended to sing songs with high entropy variance tended to also produce songs with greater energy (Figure 7A; R2 = 0.390, βstandardized = 0.533, ΔF(1,19) = 12.144, p = 0.002). This finding was not observed for females during week 1 of T-treatment (βstandardized = 0.086, p = 0.737). Likewise this finding was not observed during week 3 of T-treatment for males or females (βstandardized Male = -0.249, p = 0.549; βstandardized Female = 0.374, p = 0.147).
Figure 7.
Correlation of mean vocalization energy with mean vocalization entropy variance and mean vocalization energy with the mean total song count in males and females during week 1 and week 3. Closed circles represent male mean values and open circles represent female mean values. Trend lines for males are solid lines and dashed lines for females. Significant trend lines are highlighted in grey. A) For males during week 1 there is a significant linear relationship between the loudness of a vocalization and its acoustic stability with louder vocalizations being having higher entropy variance, which is, associated with less stability (i.e. less stereotyped vocalizations). B) Furthermore, there was a striking sex and week interaction where for males only during week 3 we observed that the greater the vocalization energy the greater the mean total number of bouts per recording.
Furthermore, there was a distinct sex difference that emerged during week 3 of treatment where it was found that in males the greater the vocalization energy the greater the mean total number of bouts per observation (Figure 7B; R2 = 0.504, βstandardized = 0.710, ΔF(1,18) = 18.273, p < 0.001). Thus males who sang loudly sang a greater total number of songs per sampling period. This finding was not observed in females during week 3 of treatment (βstandardized = 0.099, p = 0.596). In addition, during week 1 of treatment neither males nor females demonstrated this relation between energy and the mean total number of bouts per recording (βstandardized Male = 0.141, p = 0.328; βstandardized Female = 0.162, p = 0.546).
3.8. Song learning: syllable development
As described in the methods, we tracked the development of syllables and cross-correlated binary template sonograms of high amplitude song elements produced on the final day of treatment with sonograms of high amplitude syllables produced on days 5, 10, 15, and 20. High amplitude syllables were randomly re-sampled for each individual day [i.e. days 5, 10, 15, and 20] prior to cross-correlation to control for selection bias. We then calculated the percentages of these syllables in which the sonogram correlation was 0.95 or higher. These data were then analyzed in a 3-way split-plot factorial ANOVA (i.e. 3-way mixed-design with 2 between [i.e. sex and dose] and 1 within subjects variable [i.e. sampling day 5, 10, 15, or 20]). These data were only collected from birds sacrificed at 3-weeks.
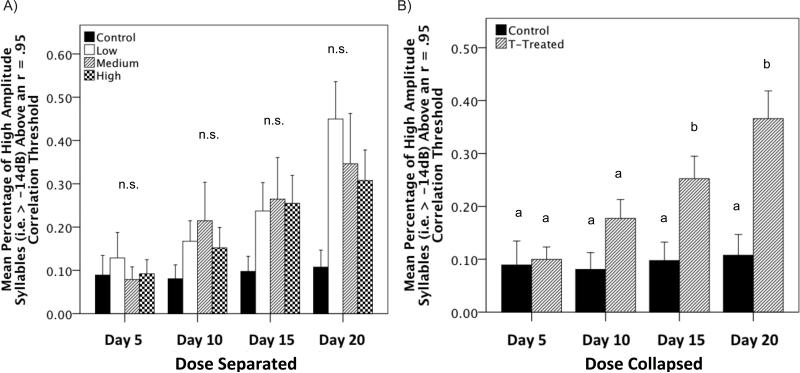
We found that there was a significant effect of sampling day on the mean percentage of high amplitude syllables above a r = 0.95 correlation threshold (Figure 8A; F(3,102) = 16.037, p < 0.001, η2 = 0.321). There was no significant main effect of sex (F(1,34) = 0.475, p = 0.495). Unexpectedly, there was no significant main effect of dose (F(3,34) = 1.748, p = 0.176) despite the mean percentage of syllables above threshold remaining constant at approximately 10% for control birds across sampling days. However, there was an interaction between the sampling day and dose, but, after Greenhouse-Geisser correction for non-sphericity the finding did not meet criterion for significance (F(3,102) = 2.049, p = 0.054). All other interactions were also not significant (Fsex*dose(3,34) = 0.299, p = 0.826; Fday*sex(3,102) = 0.399, p = 0.718; Fday*sex*dose(9,102) = 0.413, p = 0.902). Post-hoc trend analysis revealed a significant linear trend where syllables were more highly correlated as the duration of treatment increased (F(1,38) = 32.590, p < 0.001). Furthermore, for this measure there was a large amount of variance between individuals. This large amount of variance did not provide enough parametric space to fully elucidate the differences between the birds treated with T and the blank treated control birds. To account for significant individual variation in the effect of dose on this measure we combined the low, medium, and high groups and re-analyzed the data comparing T-treated versus blank controls (Figure 8B).
Figure 8.
Mean percentage of syllables above a correlation threshold of r = 0.95. Across seasons male canaries drop and add new syllables to their repertoire (Nottebohm et al., 1986). This seasonal modulation of syllable development is regulated by cyclical changes in endogenous T (Nottebohm et al., 1986). Treatment with exogenous T induced singing and syllable development/refinement in both males and females. The pattern of syllable development did not differ between males and females. A) Furthermore, the pattern did not differ as a function of the dose of T received and B) when collapsed into T-treated versus blank controls it is clear that syllables become more stereotyped in the T-treated birds relative to controls. Bars with a same letter are not significantly different; bars with a different letter are different for p≤0.05 in the post-hoc tests. Bars marked with an ‘a’ are significantly lower than bars marked with ‘b’. Likewise, bars marked with ‘a,b’ are not significantly different from bars marked ‘a’ or ‘b’. n.s.= no significant difference for the analysis of mean percentage of syllables above a correlation threshold.
As with the first analysis we found a significant effect of sampling day on the mean percentage of high amplitude syllables above a r = 0.95 correlation threshold (F(3,114) = 6.483, p < 0.001, η2 = 0.146). Likewise, there was no main effect of sex (F(1,38) = 0.815, p = 0.372). However, unlike the previous analysis there was a significant main effect of treatment where T-treated birds had a higher mean percentage of high amplitude syllables above an r = 0.95 correlation threshold (F(1,38) = 5.279, p = 0.027, η2 = 0.122). There was no interaction between sex and dose (F(1,38) = 0.230, p = 0.634). However, there was a significant interaction between sampling day and T-treatment on the mean percentage of syllables above an r = 0.95 correlation threshold (F(3,114) = 4.472, p = 0.008, η2 = 0.105). All other interactions were not significant (Fday*sex(3,102) = 0.288, p = 0.806; Fday*sex*dose(9,102) = 0.566, p = 0.615). A Bonferroni post-hoc test revealed that on sample day 5 there was no effect of treatment (F(1,42) = 0.766, p = 0.387, η2 = 0.013), by day 10 there was a non-significant trend for T-treated birds to have a greater mean percentage of syllables above threshold (F(1,42) = 3.088, p = 0.087, η2 = 0.074) and on days 15 and 20 this effect was significant (Fday15 (1,42) = 5.434, p = 0.025, η2 = 0.116; Fday20 (1,42) = 8.141, p = 0.007, η2 = 0.173).
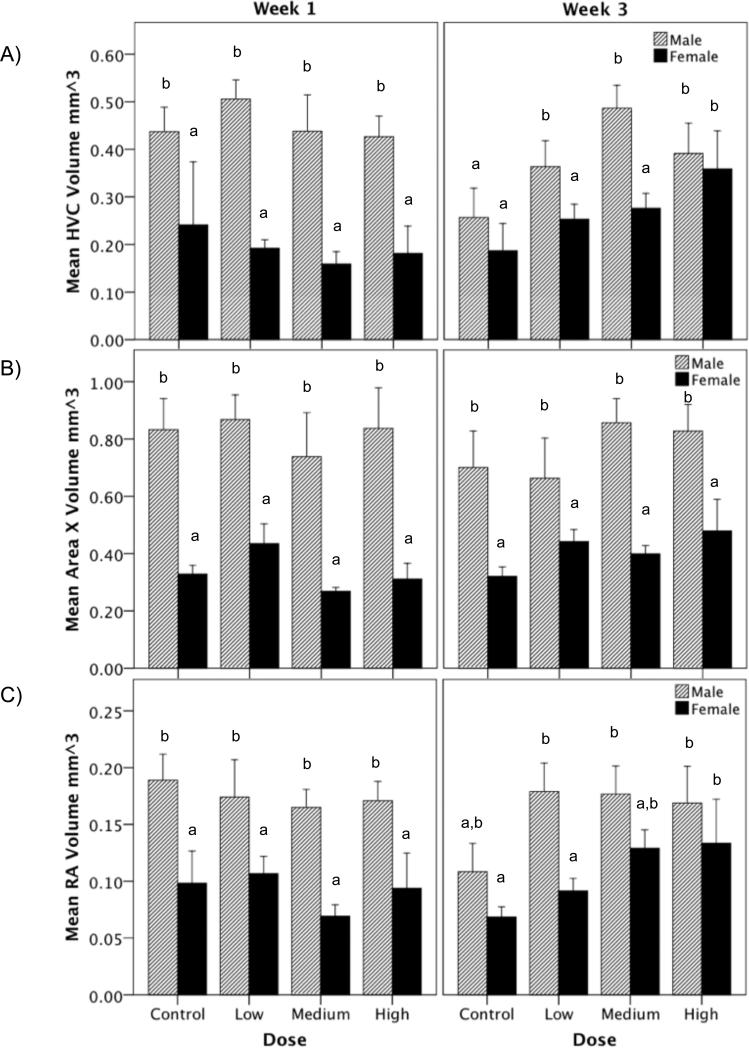
3.9. Song control nuclei volumes
One of the fundamental aims of this experiment was to assess whether there are differential responses in the brain of males and females to a same range of T doses and how these changes relate to the behavioral changes we observe. As previously mentioned, we measured the volumes of the following song control nuclei: HVC (used as a proper name), Area X, and the robust nucleus of the arcopallium (RA; Figure 9A-C). We found that for all three song control nuclei there was a robust main effect of sex where males had larger nuclei volumes compared to females (FHVC (1,73) = 42.264, p < 0.001, η2 = 0.531; FAreaX (1,73) = 74.090, p < 0.001, η2 = 0.576; FRA (1,73) = 13.303, p < 0.001, η2 = 0.328). Surprisingly, however, the main effect of dose was not significant (FHVC (1,73) = 0.899, p = 0.448, η2 = 0.220; FAreaX(3,73) = 0.478, p = 0.699, η2 = 0.100; FRA(3,73) = 0.962, p = 0.417, η2 = 0.092). Though there was no significant main effect of week FHVC (1,73) = 0.058, p = 0.811, η2 = 0.003; FAreaX (1,73) = 0.797, p = 0.376, η2 = 0.005; FRA (1,73) = 1.919, p = 0.171, η2 < 0.001) there was a significant interaction of dose and week (FHVC (1,73) = 3.930, p = 0.013, η2 = 0.208; FAreaX (1,73) = 3.030, p = 0.037, η2 = 0.181; FRA (1,73) = 3.416, p = 0.023, η2 = 0.105). The interaction of sex and dose was not significant (FHVC (3,73) = 0.809, p = 0.494, η2 = 0.059; FAreaX (3,73) = 0.371, p = 0.774, η2 = 0.013; FRA (3,73) = 0.202, p = 0.894, η2 = 0.023). The interaction of sex and week was not significant though there was a trend toward significance for HVC and RA (FHVC (1,73) = 3.898, p = 0.053, η2 = 0.136; FAreaX (1,73) = 0.513, p = 0.477, η2 = 0.013; FRA (1,73) = 3.080, p = 0.085, η2 = 0.81). The interaction of sex, dose, and week was also not significant (FHVC (3,73) = 0.986, p = 0.406, η2 = 0.104; FAreaX (3,73) = 1.212, p = 0.314, η2 = 0.083; FRA (3,73) = 0.257, p = 0.856, η2 = 0.029).
Figure 9.
Song control nuclei volumes. Overall, males had larger volumes of song control nuclei for nucleus HVC (used as its proper name; A), Area X (B), and robust nucleus of the arcopallium (abbreviated RA; C) compared to females. T also increased the volumes of HVC and RA on week 3 only. Bars with a same letter are not significantly different; bars with a different letter are different for p≤0.05 in the post-hoc tests. Bars marked with an ‘a’ are significantly lower than bars marked with ‘b’. Likewise, bars marked with ‘a,b’ are not significantly different from bars marked ‘a’ or ‘b’.
Post-hoc tests focusing on the dose by week interaction revealed that in brains collected at the end week 1, there was no significant main effect of T (FHVC (3,34) = 0.986, p = 0.415; FAreaX (3,34) = 2.600, p = 0.074; FRA (3,34) = 0.571, p = 0.639). However, in brains collected at the end of week 3 the main effect of dose was significant for HVC and RA, but not for Area X (FHVC (3,39) = 4.101, p = 0.015; FRA (3,39) = 3.898, p = 0.018; FAreaX (3,39) = 1.345, p = 0.278). Further tests showed that the effect of dose was most significant in the comparison of the medium dose versus control (HVC: i-jcontrol-medium = 0.196, p = 0.011; RA: i-jcontrol-medium = 0.093, p = 0.018); the difference between high dose and control was not significant after Bonferonni correction for multiple comparisons though there was a statistical tendency for RA (i-jcontrol-high = 0.075, p = 0.097).
4. Discussion
4.1. Testosterone treatment and the induction of song
In this experiment, we investigated how increasing doses of T affected song behavior, and how rapidly they affected this behavior and its quality in adult male and female canaries housed under the same photoperiodic conditions. Serum testosterone concentrations increased in direct relationship with the size of the implant and were clearly sufficient to markedly increase the singing rate of castrated male and intact female canaries (Figure 1). All birds treated with T increased rates of singing relative to blank treated controls (Figure 2). Interestingly, however, even birds treated with the low dose of T sang significantly more frequently than controls during week 1 and week 3 of observation. This finding is quite interesting given that birds treated with this low dose of T did not display a statistically significant rise in circulating testosterone as compared to controls. One implication of these data is that slight increases in T (increases so small that they are not distinguished by our assays) are all that is required to initiate the induction of singing. We also do not know if there has been a larger relative increase in T in the birds receiving the low dose as compared to the controls since we did not collect samples prior to treatment in this study.
4.2. Sex differences in T-induction of song
It is important to remember when considering the effects of equal doses of T on song in male and female canaries that endogenous concentrations of T are different between the sexes and that the quality and pattern of song behavior is also different. Our studies of captive male and female canaries have shown that males exhibit a clear increase in T circulating concentrations in response to long days that declines when the birds go photoreractory while female serum T concentrations never exceed concentrations measured in photosensitive males held on short days (Hurley et al., 2008). Pesch and Güttinger (1985) studied spontaneous song in socially isolated adult female canaries. They found that after the end of the reproductive period female isolates would occasionally sing. Their song was more variable than in males but shared some male-typical characteristics. They found no change in circulating steroids in association with this seemingly seasonal behavior. Since the females were isolated, it was not clear what function the behavior served especially given the fact that group housed females were not observed singing and it was thought based on older studies that females only engaged in call-like vocal behavior (Mulligan and Olsen 1969). Thus in our studies of sex differences in the ability of T to induce song we are challenging the females with doses that are higher than they normally experience. These studies will provide us with information on the degree to which female canaries can be fully sex-reversed, i.e. the degree to which they can engage in the full array of male-typical song if they are experiencing T concentrations similar to what males experience.
Both sexes did exhibit increases in the rate of singing and characteristics of song, but these effects were usually associated with different response latencies in males and females. In the case of song rate (Figure 2), males responded with a shorter latency to T treatment: during week 1, males spent a greater absolute proportion of their time singing compared to females. However, by week 3 there was no difference between male and females in the proportion of time-spent singing. Likewise, for the ratio of time spent singing versus calling (i.e. general vocalizing; Figure 3) there was a robust sex-based difference during week 1 that was no longer present during week 3. Furthermore, we see a similar pattern of response latency in the T-induced modulation of song characteristics, in this case, song bout length (Figure 4), number of notes (Figure 5), and energy (i.e. loudness; Figure 6). There was for example no significant difference between short and long term T-treatment (i.e. 1 vs. 3 weeks) on song bout length in males whereas this was the case in females. Furthermore, during week 1 of T-treatment, males sang louder songs with more syllable iterations (i.e. number of notes above detection threshold) compared to females; however, during week 3 of treatment this difference between males and females had disappeared.
These findings are quite intriguing particularly in light of the correlations that were identified in males only (Figure 7). Early in T-treatment males who sang loudly sang songs that were less stereotyped (i.e. high entropy variance) and later in T-treatment males who sang loudly tended to produce more songs in total. For male songbirds, song serves a dual function; mate attraction and defense display against other (typically male) conspecifics. T-treated males increased both rate and gain (i.e. mean energy) with short latency relative T-treated females; however this occurred prior to the refinement and crystallization of song as males who sang loudly sang less stereotyped songs (as noted by mean entropy variance). Additionally, males who sang at a high rate (i.e. high mean total song count) tended to do so with increased gain later in T-treatment (i.e. louder songs during week 3). These data demonstrate that T can activate behavioral plasticity in both males and females with differential patterns in the latency of response in correlation with specific features of song. Longer exposure times to testosterone or perhaps supraphysiolgical levels of T (relative to naturally occurring male levels) may be required in females to immediately engage the full behavioral suite as expressed by male canaries early in T-treatment (i.e. week 1).
However, we also cannot exclude the influence of social context on the behavior patterns we observe, as birds were isolated both visually and aurally from other conspecifics. Likewise, these birds were adults and presumably the males in particular had singing experience prior to this manipulation; song activity itself has been shown to have stimulatory effects on measures of neuroplasticity such as new neuron incorporation in the brain of male songbirds (Alvarez-Borda and Nottebohm, 2002). It is therefore conceivable that previous experience with song production may induce an acute sensitivity to steroid action in adulthood. This difference between males and females could underlie a good amount of the behavioral differences we observed early in our manipulation. It is also quite possible that T is acting by different neuroendocrine or neurochemical mechanisms in females relative to males and that the temporal discordance in latency we observe in the induction of song reflects this difference.
In the transition from the photosensitive to photostimulated state, males show a quick and robust change in behavior. In wild male canaries these changes can occur even before the onset of increasing day length given particular social and environmental contexts (Leitner et al., 2003). These changes in behavior are related in part to changes (in this case increases) in gonadal steroid concentration and to the rapid response of males to these endogenous and exogenous factors (i.e. T and socio-environmental context; Leitner et al., 2003). It is advantageous, in terms of reproductive fitness, for male canaries to rapidly and robustly respond behaviorally to even slight increases in T. These selective pressures are presumably different in females who do not sing as much as males and whose song is under a different type of stimulus control. It is plausible that these actions of T on behavior have not been fine tuned during evolution in this sex.
4.3. Similarities in syllable development in males and females
In temperate zone songbird species that are open-ended learners, seasonal shifts in circulating T induce the recapitulation of sensorimotor song learning and the crystallization of new arrangements (Nottebohm, 1984). Song develops in two phases, a sensory phase (auditory memory formation in juveniles) and a sensorimotor phase (plastic song produced and refined to crystallized song; reviewed in Marler, 1997 and Ball and Hulse, 1999). In some species like canaries, it has been shown in captive populations that, across seasons, adult birds can drop syllables through selective attrition or add new syllables (Nottebohm et al, 1986). Likewise, in adult canaries the sequencing of song(s) (i.e. the arrangement of the individual syllables and phrases) can change across seasons (Leitner et al, 2001). To evaluate sensorimotor learning and the development of syllables, we cross-correlated high amplitude song elements produced on the final day of treatment with high amplitude syllables on days 5, 10, 15, and 20 (Figure 8). We found that there was a significant main effect of sampling day on the mean percentage of high amplitude syllables above an r = 0.95 correlation threshold. Surprisingly, there was no difference between the effects of the different doses of T given (i.e. low, medium, and high) on syllable development. When the groups were collapsed to T versus blank controls it was clear that T-treated birds exhibited a linear progression in the development of syllables with time, a progression that was not observed in control birds. There was thus clearly an effect of T on this song feature but the lowest dose was already fully effective for increasing song stereotypy.
High amplitude individual syllable iterations also became more stereotyped at the same rate in males and females indicating a similar pattern of T-induced syllable crystallization in both sexes (Figure 8). This finding stands in contrast to the sex differences observed in the increases of song rate (Figure 2) and other song characteristics (Figures 4-6). T can thus affect some song features in a sex-specific manner whereas other features display similar responses following exposure to the steroid. This discrepancy points to the existence of divergent underlying mechanisms and possibly divergent selective pressures that organized the brain reaction to steroids in a sex-specific manner. It also supports our previous suggestion (Ball et al., 2008) that effects of T on the motivation and readiness to sing (reflected here in the song rate and probably song energy) are not necessarily mediated by the same mechanisms nor by an action in the same brain region as effects on song learning and song quality (measured here by syllable stereotypy). This separation of T action sites on different features of song has now been experimentally demonstrated (Alward et al., 2013).
4.4. Song control nuclei volumes
It was initially demonstrated that the vernal increase in serum T concentrations observed during the annual cycle correlates with increases in the volume of song control nuclei such as HVC, RA, and area X (Nottebohm et al., 1987). Multiple studies later showed that treatment with exogenous T of photoregressed or gonadectomized subjects of both sexes largely mimics this effect therefore demonstrating the causal role of T in this aspect of neuroplasticity (Sartor et al., 2005; Smith et al., 1997; Strand and Deviche, 2007). However, no study has to our knowledge directly tested whether identical treatments with exogenous T have identical effects on the morphology of the song-control system in both male and female canaries.
We found here that even after treatment with a range of doses of T there is still a robust sex difference in the volume of song control nuclei so that males have much larger volumes of HVC, RA, and area X than females (Figure 9). In this study, we failed to identify a significant overall effect of T on the volume of these nuclei. However, there was a significant interaction of dose and week resulting from the fact that by week 3, birds treated with T had larger HVC and RA (but not Area X) volumes compared to controls whereas such effects were not seen on week 1. An explanation for this unexpected pattern of results is suggested by the observation that in control males and females, the volume of all 3 nuclei decreased, sometimes very markedly (e.g. for HVC and RA in males) between week 1 and week 3. This overall decrease in the volume of the song control nuclei may be the result of the birds being moved at the start of the experiment (at the beginning of T treatment) from group housing to individual cages in sound attenuated chambers. Placement into individual cages was implemented in order to obtain high quality sound recordings. We suggest that by week 1 this stress associated with the transfer to new housing conditions was the most intense and its effects were prominent enough to block the stimulatory effects of T but stress had not yet exerted a major inhibitory effect on the size of song control nuclei (although this cannot be directly assessed since no volume measures are available before the beginning of the experiment). By week 3, both factors had exerted their effects so that volumes had decreased in the control group while T had compensated that effect in the T-treated groups in a dose-dependent manner.
This scenario is made quite likely by a previous MRI study that allowed investigators to measure, within subjects, changes over time in song nuclei volume; they found that RA volume decreased in female starlings after transfer from a group housing condition to an individual housing condition (van Meir et al., 2004). Although not directly measured in the van Meir et al. (2004) study nor in the present study, housing the birds in isolation presumably causes an activation of the stress axis. Previous studies have demonstrated that songbirds show a decrease in the number of new neurons (Lipkind et al., 2002) and smaller HVC volumes (Leitner & Catchpole, 2007) in response to social isolation. Thus, social isolation may be perceived as stressful to this social species, resulting in the activation of the stress axis.
The primary glucocorticoid released in birds is corticosterone (CORT) and there is evidence that CORT can decrease the volume of song control nuclei such as HVC, namely by inhibiting neurogenesis (Newman et al., 2010). Under these conditions, T did not produce an overall increase in volume of song control nuclei that has been reported in previous canary studies (Appeltants et al., 2003; Nottebohm, 1980; Sartor et al., 2005) but rather counter-acted the stress-induced decrease.
The time course of T action that would compensate stress effects after only 3 weeks is compatible with previously published data collected in white-crowned sparrows. Treatment of captive white-crowned sparrows held under long day conditions with exogenous T that increased plasma concentrations to 4-10 ng/ml did result in an increase in HVC volume within one week (Tramontin et al., 2000). However, we maintained our birds on short days and birds with the largest implants had T concentrations of about 2 ng/ml, which reflects the highest concentrations we had measured in male canaries previously (Hurley et al., 2008). Field studies of white-crowned sparrows have now demonstrated that T concentrations below 2ng/ml are sufficient to induce growth of the song system in these birds albeit with a time course longer than 1 week (Tramontin et al., 2001).
Furthermore, field studies by Leitner and colleagues (2001) revealed significant changes in the song behavior of wild male canaries in association with changes in circulating T without seeing significant differences in the volumes of HVC and RA at these same time points. Considering our data in light of these findings, a strong case can be made for T acting through multiple ways to support changes in song behavior. We have previously shown that T acting only in the POM can induced increases in song rate in male canaries (Alward et al., 2013). Changes in the volume of song control nuclei may not be necessary to induce changes in behavior. Changes to the state or neurochemical profile of neurons in the song system as well as changes in other areas outside of the song system may equally be likely to induce behavioral changes..
4.5. Relationships between song control nuclei volumes and singing behavior
The comparison of these somewhat atypical volumetric changes with the behavioral effects described before leads to a number of very interesting conclusions. First, we demonstrate here that a) a major increase in song rate, vocalization energy and song stereotypy can take place in the absence of overall increases in the volume of HVC or RA (during week 1) and furthermore that b) these behavioral changes occurred for the most part while HVC and RA volumes were decreasing. These observations bring additional support to the notion that effects of T on singing behavior are taking place at multiple brain sites and in particular that the motivation to sing is likely controlled outside the song system, possibly at the level of the medial preoptic area (see Ball and Balthazart, 2008; Ball et al., 2003; Riters and Ball, 1999).
Secondly, the present studies also demonstrate that many, but not all, aspects of the sex differences in singing behavior disappear, at various rates, when males and females are placed in a similar endocrine environment while at the same time sex differences in the volume of song control nuclei are maintained. This brings additional support to the notion that other brain areas must be involved in song control and/or that the volume of song nuclei represents a useful but incomplete measure of their functional potential.
The persistence of sex differences in volumes after 3 weeks of exposure to a similar endocrine environment additionally raises the question of the origin of these differences. In birds and mammals, sex steroids early in development (i.e. ontogeny) act to organize the brain in a sex-typical fashion and later in adulthood they act to activate these sex-typical pathways supporting the expression of sexually differentiated behaviors (Arnold and Gorski, 1984; Goy and McEwen, 1979; McCarthy et al., 2012; Phoenix et al., 1959). Although the sexual differentiation of the song control system remains poorly understood (Wade and Arnold, 2004) and there has been no work on this phenomenon in canaries (most work was performed on zebra finches; Arnold and Gorski, 1984; Adkins-Regan, et al., 1994; Wade and Arnold, 1996; and only one study on starlings; Casto and Ball 1996), female canaries are presumably not exposed to the same endocrine conditions during ontogeny as males. Female canaries were probably never exposed to high T concentrations before this experiment, contrary to males. It is therefore conceivable that previous exposure to T organized the brains of males to respond to T action in adulthood with acute sensitivity. It could also be argued that a 3 week-period was too short to produce the full complement of T effects in the female brain and that the volumetric sex differences observed on week 3 only reflect a different rate of response so that females would after a longer period reach the same condition as males. There is however no indication in the available data that this would be the case (sex differences are in general as large on week 3 as on week 1) and only additional longer experiments could answer this question.
Alternatively, it has been demonstrated in zebra finches that volumetric sex differences in the song system are controlled in part by genetic sex differences that act independently of circulating concentrations of sex steroids (Agate et al., 2003). The contribution of similar mechanisms is obviously conceivable, if not probable, in canaries and the sex differences in HVC, RA and Area X volumes observed here in T treated birds might reflect this phenomenon.
In conclusion, the present studies show that treatment of castrated male and photo-regressed female canaries with a range of doses of T ultimately results in both sexes showing a relatively similar male-typical singing behavior (after 3 weeks of treatment) but that males respond faster than females. Surprisingly there was little effect of T on the song control nuclei volumes of females and the volume increases were observed in males only after 3 weeks of treatment whereas very pronounced, nearly maximal, changes in singing behavior were already observed on week 1. Song rate and song structure can thus be affected by T in male and female canaries in the absence of the usually associated changes song control nuclei volumes.
Manuscript Highlights.
- Exogenous T induces relatively similar singing behavior in male and female canaries
- Song rate and other song features respond faster to T in males than females
- T-induced changes in singing precede changes in the volume of song control nuclei
- Singing in female is not associated with male-typical song control nuclei volumes
- Song can be affected by T in the absence of changes in song control nuclei volumes
Acknowledgements
We would like to thank our undergraduate assistant Janice Rivelle for helping with song analysis, our lab manager Wade Mayes for helping with the Thionin staining and set up of the recording system, and Jim Garmon for constructing the sound attenuated chambers. This work was funded by NIH/NINDS RO1 35467 to GFB and JB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have nothing to disclose.
References
- Adkins-Regan E, Mansukhani V, Seiwert C, Thompson R. Sexual-differentiation of brain and behavior in the zebra finch- critical periods for effects of early estrogen-treatment. Journal of Neurobiology. 1994;25:865–877. doi: 10.1002/neu.480250710. [DOI] [PubMed] [Google Scholar]
- Agate RJ, Grisham W, Wade J, Mann S, Wingfield J, Schanen C, Palotie A, Arnold AP. Neural, not gonadal, origin of brain sex differences in a gynandromorphic finch. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4873–4878. doi: 10.1073/pnas.0636925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Borda B, Nottebohm F. Gonads and singing play separate, additive roles in new neuron recruitment in adult canary brain. The Journal of neuroscience. 2002;22(19):8684–8690. doi: 10.1523/JNEUROSCI.22-19-08684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alward BA, Balthazart J, Ball GF. Differential effects of global versus local testosterone on singing behavior and its underlying neural substrate. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:19573–19578. doi: 10.1073/pnas.1311371110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appeltants D, Ball GF, Balthazart J. Song activation by testosterone is associated with an increased catecholaminergic innervation of the song control system in female canaries. Neuroscience. 2003;121:801–814. doi: 10.1016/s0306-4522(03)00496-2. [DOI] [PubMed] [Google Scholar]
- Arai O, Taniguchi I, Saito N. Correlation between the size of song control nuclei and plumage color-change in orange bishop birds. Neuroscience Letters. 1989;98:144–148. doi: 10.1016/0304-3940(89)90500-4. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annual review of neuroscience. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- Baker MC, Spitler-Nabors KJ, Bradley DC. Early experience determines song dialect responsiveness of female sparrows. Science. 1981;214:819–821. doi: 10.1126/science.214.4522.819. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Individual variation and the endocrine regulation of behaviour and physiology in birds: a cellular/molecular perspective. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2008;363:1699–1710. doi: 10.1098/rstb.2007.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball GF, Bernard DJ, Foidart A, Lakaye B, Balthazart J. Steroid sensitive sites in the avian brain: does the distribution of the estrogen receptor alpha and beta types provide insight into their function? Brain, Behavior and eEvolution. 1999;54:28–40. doi: 10.1159/000006609. [DOI] [PubMed] [Google Scholar]
- Ball GF, Castelino CB, Maney DL, Appeltants D, Balthazart J. The activation of birdsong by testosterone: multiple sites of action and role of ascending catecholamine projections. Annals of the New York Academy of Sciences. 2003;1007:211–231. doi: 10.1196/annals.1286.021. [DOI] [PubMed] [Google Scholar]
- Ball GF, Hulse SH. Birdsong. American Psychologist. 1998;53(1):37. doi: 10.1037//0003-066x.53.1.37. [DOI] [PubMed] [Google Scholar]
- Ball GF, Ketterson ED. Sex differences in the response to environmental cues regulating seasonal reproduction in birds. Philosophical transactions of the Royal Society of London. Series B. Biological sciences. 2008;363:231–246. doi: 10.1098/rstb.2007.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball GF, Riters LV, MacDougall-Shackleton SA, Balthazart J. Sex differences in brain and behavior and the neuroendocrine control of the motivation to sing. In: Zeigler HP, Marler PR, editors. The Neuroscience of Birdsong. Cambridge University Press; Cambridge, UK: 2008. pp. 320–331. [Google Scholar]
- Bentley GE, Van't Hof TJ, Ball GF. Seasonal neuroplasticity in the songbird telencephalon: a role for melatonin. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4674–4679. doi: 10.1073/pnas.96.8.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard DJ, Ball GF. Two histological markers reveal a similar photoperiodic difference in the volume of the high vocal center in male European starlings. The Journal of Comparative Neurology. 1995;360:726–734. doi: 10.1002/cne.903600415. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Ball GF. Photoperiodic condition modulates the effects of testosterone on song control nuclei volumes in male European starlings. General and Comparative Endocrinology. 1997;105:276–283. doi: 10.1006/gcen.1996.6829. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Nalls B, Wingfield JC, Kroodsma DE. Seasonal changes in avian song nuclei without seasonal changes in song repertoire. The Journal of Neuroscience. 1991;11:1367–1374. doi: 10.1523/JNEUROSCI.11-05-01367.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumm H, Todt D. Noise-dependent song amplitude regulation in a territorial songbird. Animal Behaviour. 2002;63:891–897. [Google Scholar]
- Brumm H, Todt D. Male-male vocal interactions and the adjustment of song amplitude in a territorial bird. Animal Behaviour. 2004;67:281–286. [Google Scholar]
- Catchpole CK. Sexual selection and the evolution of complex songs among European warblers of the genuc acrocephalus. Behaviour. 1980;74:149–166. [Google Scholar]
- Catchpole CK. The evolution of bird sounds in relation to mating and spacing behavior. In: Kroodsma DE, Miller EH, editors. Acoustic communication in birds. Vol. 1. Academic Press; New York: 1982. pp. 297–319. [Google Scholar]
- Catchpole CK, Slater PJB. Birdsong: Themes and Variations. 2nd edition. Cambridge University Press; New York: 2008. [Google Scholar]
- Casto JM, Ball GF. Early administration of 17beta-estradiol partially masculinizes song control regions and alpha2-adrenergic receptor distribution in European starlings (Sturnus vulgaris). Hormones and Behavior. 1996;30:387–406. doi: 10.1006/hbeh.1996.0044. [DOI] [PubMed] [Google Scholar]
- Gahr M, Garcia-Segura LM. Testosterone-dependent increase of gap-junctions in HVC neurons of adult female canaries. Brain Research. 1996;712:69–73. doi: 10.1016/0006-8993(95)01448-9. [DOI] [PubMed] [Google Scholar]
- Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proceedings of the National Academy of Sciences. 1983;80(8):2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goy RW, McEwen BS. Sexual Differentiation of the Brain. MIT Press; Cambridge, MA: 1979. [Google Scholar]
- Güttinger HR. Consequences of domestication on the song structures in the canary. Behaviour. 1985:254–278. [Google Scholar]
- Güttinger HR, Prove E, Weichel K, Pesch A. Sex steroids and development of song in the canary. Journal Fur Ornithologie. 1984;125:245–247. [Google Scholar]
- Güttinger HR, Wolffgramm J, Thimm F. Relationship between species-specific song programs and individual learning in songbirds – Study of individual variation in songs of canaries, greenfinches, and hybrids between two species. Behaviour. 1978;65:241–262. [Google Scholar]
- Harding CF. Hormonal modulation of singing: hormonal modulation of the songbird brain and singing behavior. Annals of the New York Academy of Sciences. 2004;1016:524–539. doi: 10.1196/annals.1298.030. [DOI] [PubMed] [Google Scholar]
- Hartog TE, Dittrich F, Pieneman AW, Jansen RF, Frankl-Vilches C, Lessmann V, Lilliehook C, Goldman SA, Gahr M. Brain-derived neurotrophic factor signaling in the HVC is required for testosterone-induced song of female canaries. The Journal of Neuroscience. 2009;29:15511–15519. doi: 10.1523/JNEUROSCI.2564-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LL, Wallace AM, Sartor JJ, Ball GF. Photoperiodic induced changes in reproductive state of border canaries (Serinus canaria) are associated with marked variation in hypothalamic gonadotropin-releasing hormone immunoreactivity and the volume of song control regions. General and Comparative Endocrinology. 2008;158:10–19. doi: 10.1016/j.ygcen.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AP, West MJ. Species identification in the North American cowbird: appropriate responses to abnormal song. Science. 1977;195:1002–1004. doi: 10.1126/science.841321. [DOI] [PubMed] [Google Scholar]
- Kirn JR, Clower RP, Kroodsma DE, Devoogd TJ. Song-related brain regions in the red-winged blackbird are affected by sex and season but not repertoire size. Journal of Neurobiology. 1989;20:139–163. doi: 10.1002/neu.480200304. [DOI] [PubMed] [Google Scholar]
- Krebs JR. Significance of song repertoires – The Beau Geste hypothesis. Animal Behaviour. 1977;25:475–478. [Google Scholar]
- Kroodsma DE. Reproductive development in a female songbird: differential stimulation by quality of male song. Science. 1976;192:574–575. doi: 10.1126/science.192.4239.574. [DOI] [PubMed] [Google Scholar]
- Leitner S, Catchpole CK. Female canaries that respond and discriminate more between male songs of different quality have a larger song control nucleus (HVC) in the brain. Journal of Neurobiology. 2002;52:294–301. doi: 10.1002/neu.10085. [DOI] [PubMed] [Google Scholar]
- Leitner S, Catchpole CK. Song and brain development in canaries raised under different conditions of acoustic and social isolation over two years. Developmental Neurobiology. 2007;67:1478–1487. doi: 10.1002/dneu.20521. [DOI] [PubMed] [Google Scholar]
- Leitner S, Van't Hof TJ, Gahr M. Flexible reproduction in wild canaries is independent of photoperiod. General and comparative endocrinology. 2003;130:102–108. doi: 10.1016/s0016-6480(02)00574-9. [DOI] [PubMed] [Google Scholar]
- Leitner S, Voigt C, Garcia-Segura LM, Van't Hof T, Gahr M. Seasonal activation and inactivation of song motor memories in wild canaries is not reflected in neuroanatomical changes of forebrain song areas. Hormones and Behavior. 2001;40:160–168. doi: 10.1006/hbeh.2001.1700. [DOI] [PubMed] [Google Scholar]
- Lipkind D, Nottebohm F, Rado R, Barnea A. Social change affects the survival of new neurons in the forebrain of adult songbirds. Behavioural Brain Research. 2002;133:31–43. doi: 10.1016/s0166-4328(01)00416-8. [DOI] [PubMed] [Google Scholar]
- Marler P. The voice of the Chaffinch and its function as a language. Ibis. 1956;98:231–261. [Google Scholar]
- Marler P. Three models of song learning: evidence from behavior. Journal of Neurobiology. 1997;33(5):501–516. [PubMed] [Google Scholar]
- Marler P, Peters S. Structural-changes in song ontogeny in the swamp sparrow Melospiza-georgiana. Auk. 1982;99:446–458. [Google Scholar]
- Marler P, Peters S, Ball GF, Dufty AM, Wingfield JC. The role of sex steroids in the acquisition and production of birdsong. Nature. 1988;336(6201):770–772. doi: 10.1038/336770a0. [DOI] [PubMed] [Google Scholar]
- Marler P, Waser MS. Role of auditory-feedback in canary song development. Journal of Comparative and Physiological Psychology. 1977;91:8–16. doi: 10.1037/h0077303. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: the not so inconvenient truth. The Journal of Neuroscience. 2012;32:2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan and Olsen . In: vocalizations. Hinde RA, editor. Cambridge University Press; Cambridge, England: 1969. pp. 165–-184. [Google Scholar]
- National Research Council . Guide for the Care and Use of Laboratory Animals. Eighth Edition The National Academies Press; 2011. [Google Scholar]
- Newman AE, MacDougall-Shackleton SA, An YS, Kriengwatana B, Soma KK. Corticosterone and dehydroepiandrosterone have opposing effects on adult neuroplasticity in the avian song control system. The Journal of Comparative Neurology. 2010;518:3662–3678. doi: 10.1002/cne.22395. [DOI] [PubMed] [Google Scholar]
- Nicholls TJ, Goldsmith AR, Dawson A. Photorefractoriness in birds and comparison with mammals. Physiological Reviews. 1998;68:133–176. doi: 10.1152/physrev.1988.68.1.133. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Testosterone triggers growth of brain vocal control nuclei in adult female canaries. Brain Research. 1980;189:429–436. doi: 10.1016/0006-8993(80)90102-x. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. A brain for all seasons: cyclical anatomical changes in song control nuclei of the canary brain. Science. 1981;214:1368–1370. doi: 10.1126/science.7313697. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Birdsong as a model in which to study brain processes related to learning. Condor. 1984;86(3):227–236. [Google Scholar]
- Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194:211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Nottebohm ME. Relationship between Song Repertoire and Age in the Canary, Serinus canarius. Zeitschrift für Tierpsychologie. 1978;46:298–305. [Google Scholar]
- Nottebohm F, Nottebohm ME, Crane L. Developmental and seasonal changes in canary song and their relation to changes in the anatomy of song-control nuclei. Behavioral and Neural Biology. 1986;46:445–471. doi: 10.1016/s0163-1047(86)90485-1. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Nottebohm ME, Crane LA, Wingfield JC. Seasonal changes in gonadal hormone levels of adult male canaries and their relation to song. Behavioral and Neural Biology. 1987;47:197–211. doi: 10.1016/s0163-1047(87)90327-x. [DOI] [PubMed] [Google Scholar]
- Pesch A, Güttinger HR. Der Gesang des weiblichen Kanarienvogels. Journal für Ornithologie. 1985;126(1):108–110. [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Poulsen H. Song learning in the domestic canary. Zeitschrift für Tierpsychologie. 1959;16:173–178. [Google Scholar]
- Riters LV, Ball GF. Lesions to the medial preoptic area affect singing in the male European starling (Sturnus vulgaris). Hormones and Behavior. 1999;36:276–286. doi: 10.1006/hbeh.1999.1549. [DOI] [PubMed] [Google Scholar]
- Riebel K. The “mute” sex revisited: Vocal production and perception learning in female songbirds. Advances in the Study of Behavior. 2003;33:49–86. 33. [Google Scholar]
- Sartor JJ, Balthazart J, Ball GF. Coordinated and dissociated effects of testosterone on singing behavior and song control nuclei in canaries (Serinus canaria). Hormones and Behavior. 2005;47:467–476. doi: 10.1016/j.yhbeh.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Schlinger BA. Sex steroids and their actions on the birdsong system. Journal of Neurobiology. 1997;33:619–631. [PubMed] [Google Scholar]
- Searcy WA, Nowicki S. The Evolution of Animal Communication: Reliability and Deception in Signaling Systems. Princeton University Press; 2005. [Google Scholar]
- Shank SS, Margoliash D. Sleep and sensorimotor integration during early vocal learning in a songbird. Nature. 2009;458:73–U74. doi: 10.1038/nature07615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GT, Brenowitz EA, Beecher MD, Wingfield JC. Seasonal changes in testosterone, neural attributes of song control nuclei, and song structure in wild songbirds. Journal of Neuroscience. 1997;17:6001–10. doi: 10.1523/JNEUROSCI.17-15-06001.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand CR, Deviche P. Hormonal and environmental control of song control region growth and new neuron addition in adult male house finches, Carpodacus mexicanus. Developmental neurobiology. 2007;67:827–837. doi: 10.1002/dneu.20400. [DOI] [PubMed] [Google Scholar]
- Tchernichovski O, Mitra PP, Lints T, Nottebohm F. Dynamics of the vocal imitation process: How a zebra finch learns its song. Science. 2001;291:2564–2569. doi: 10.1126/science.1058522. [DOI] [PubMed] [Google Scholar]
- Todt D, Naguib M. Slater PJB, Rosenblatt JS, Snowdon CT, Roper TJ, editors. Vocal interactions in birds: The use of song as a model in communication. Advances in the Study of Behavior. 2000;29:247–296. [Google Scholar]
- Tramontin AD, Hartman VN, Brenowitz EA. Breeding conditions induce rapid and sequential growth in adult avian song control circuits: a model of seasonal plasticity in the brain. Journal of Neuroscience. 2000;20:854–861. doi: 10.1523/JNEUROSCI.20-02-00854.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontin AD, Perfito N, Wingfield JC, Brenowitz EA. Seasonal growth of song control nuclei precedes seasonal reproductive development in wild adult song sparrows. General and Comparative Endocrinology. 2001;122:1–9. doi: 10.1006/gcen.2000.7597. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Smith GT, Breuner CW, Brenowitz EA. Seasonal plasticity and sexual dimorphism in the avian song control system: stereological measurement of neuron density and number. Journal of Comparative Neurolology. 1998;396:186–192. doi: 10.1002/(sici)1096-9861(19980629)396:2<186::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Vallet E, Kreutzer M, Gahr M. Testosterone Induces Sexual Release Quality in the Song of Female Canaries. Ethology. 1996;102:617–628. [Google Scholar]
- Van Meir V, Verhoye M, Absil P, Eens M, Balthazart J, Van der Linden A. Differential effects of testosterone on neuronal populations and their connections in a sensorimotor brain nucleus controlling song production in songbirds: a manganese enhanced-magnetic resonance imaging study. NeuroImage. 2004;21:914–923. doi: 10.1016/j.neuroimage.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Wade J, Springer M, Wingfield J, Arnold A. Neither testicular androgens nor embryonic aromatase activity alters morphology of the neural song system in zebra finches. Biology of Reproduction. 1996;55:1126–1132. doi: 10.1095/biolreprod55.5.1126. [DOI] [PubMed] [Google Scholar]
- Wade J, Arnold AP. Sexual differentiation of the zebra finch song system. Annals of the New York Academy of Sciences. 2004;1016:540–559. doi: 10.1196/annals.1298.015. [DOI] [PubMed] [Google Scholar]
- Waser MS, Marler P. Song learning in canaries. Journal of Comparative and Physiological psychology. 1977;91:1–7. doi: 10.1037/h0077303. [DOI] [PubMed] [Google Scholar]
- Whitfield-Rucker M, Cassone V. Melatonin binding in the house sparrow song control system: Sexual dimorphism and the effect of photoperiod. Hormones and Behavior. 1996;30:528–537. doi: 10.1006/hbeh.1996.0056. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Kenagy GJ. Natural regulation of reproductive cycles. In: Schreibman MP, Jones RE, editors. Vertebrate Endocrinology: Fundamentals and biomedical implications. Academic Press; New York: 1991. pp. 181–241. [Google Scholar]
- Wingfield JC, Doak D, Hahn TP. Integration of environmental cues regulating transitions of physiological state, morphology and behavior. In “Avian Endocrinology”. In: Sharp PJ, editor. Journal of Endocrinology. Bristol, UK.: 1993. pp. 111–122. [Google Scholar]
- Wolffgramm J. Sequences of vocal patterns and periodic relationships in song of roller-canary (Serinus-canaria). Journal of Comparative Physiology. 1973;85:65–88. [Google Scholar]