Figure 1. Overview of clinical study.
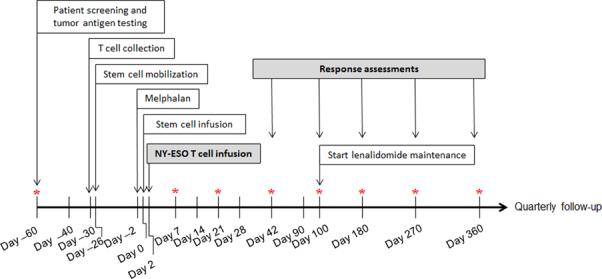
Patient screening, including HLA testing and tumor antigen testing, and apheresis scheduling requires 2-4 weeks. Manufacture of gene-modified cells takes 3-4 weeks. Patients received high dose melphalan two days prior to stem cell infusion, and four days prior to T-cell infusion. Response assessments were performed at day 42, 100, 180 and quarterly thereafter. Optional bone marrow biopsies are indicated by asterisk. For eligible patients, maintenance lenalidomide was given starting at day 100. Once off study, patients are monitored for up to 15 years for delayed adverse events in accordance with FDA Guidance.
