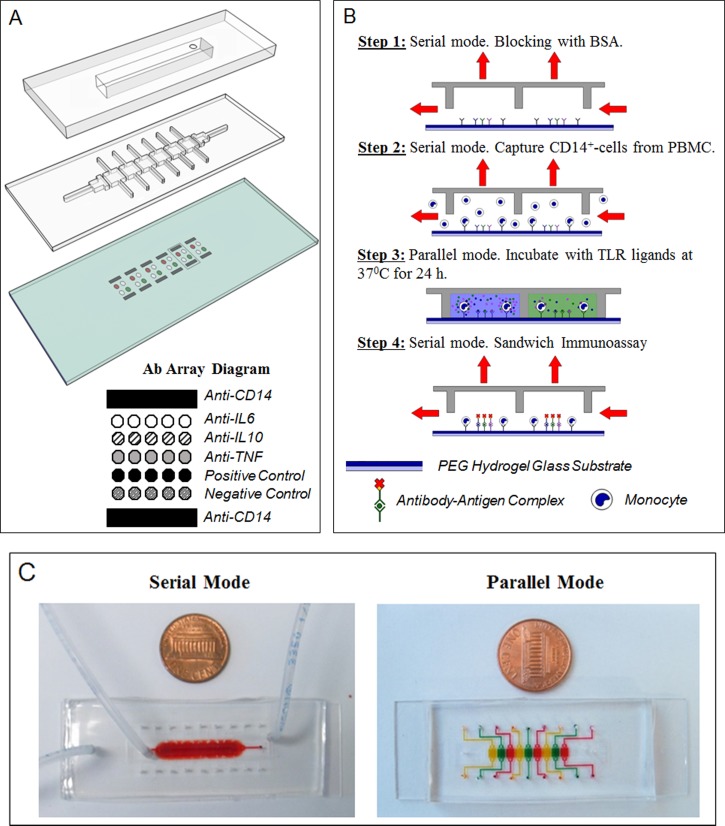
FIG. 1.
Microfluidic platform layout and operation. (a) The microfluidic platform consists of a two-layer PDMS device mounted on top of an antibody imprinted PEG substrate. A top control layer has a vacuum insertable chamber which once activated lifts up the bottom layer. The two layers work together to permit toggling between serial and parallel flow of solution. The hydrogel slide contains 6 set of antibody arrays containing IgG towards monocytes and cytokines. They were spaced based on the dimensions of each channel to allow each set of array to be comfortably enclosed by each respective PDMS chamber. Anti-CD14 cell capture spots were printed as interlaced spots to form a wide band of antibody spots adhesive to cells. Cytokine capture spots were printed between cell capture bands and consisted of IgG against IL-6, IL-10, TNF-α, biotinylated, and non-biotinylated isotype controls. (b) Overview of device operation. Step 1: Roof was lifted to create one channel (serial mode). The device was primed and incubated with BSA. Step 2: Mononuclear cells were infused into the device, with monocytes becoming captured on anti-CD14 Ab spots. Step 3: Roof was lowered creating individually addressable compartments. Each compartment contained the same number of cell capture and cytokine detection Ab spots. Each compartment was infused with a different TLR agonist to induce cytokine production from cells. Step 4: After incubation for 24 h, the roof was raised again. A mixture of biotinylated detection Abs and streptavidin conjugate were infused into the channel for immunostaining of cytokine capture spots. (c) Pictures of the device configured to operate in serial and parallel mode. Food dye was used to highlight that in serial mode, the device represents one straight channel, whereas in parallel mode it becomes 6 individually addressable compartments.