Figure 5. Kinetic Binding Model of Tropomyosin Molecules to F-actin.
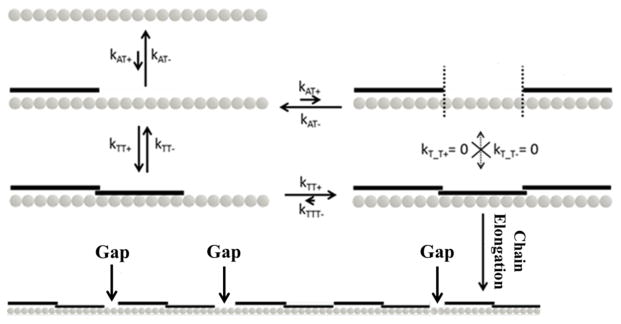
The relative quantities of various kinetic constants are represented by arrow lengths. A single tropomyosin molecule initially binds bare F-actin with low affinity. Once one binds (kAT+), a second can contiguously bind via end-to-end bond formation (kTT+), bind at a separate lattice site (kAT+), or fall off the filament (kAT−). Once two are contiguously bound, a monomer can detach (kTT−) or a third can contiguously add to the chain (kTT+). If three tropomyosin monomers contiguously bind, sustained chain growth is favored because it is unlikely that the rate of detachment from an end (kTTT−) or middle molecule (kT_T−) will exceed kTT+. The formation of multiple growth sites along the actin filament, and subsequent chain elongation from them, results in the buildup of gaps where there are not exactly seven actin monomers sandwiched between adjacent chains.
