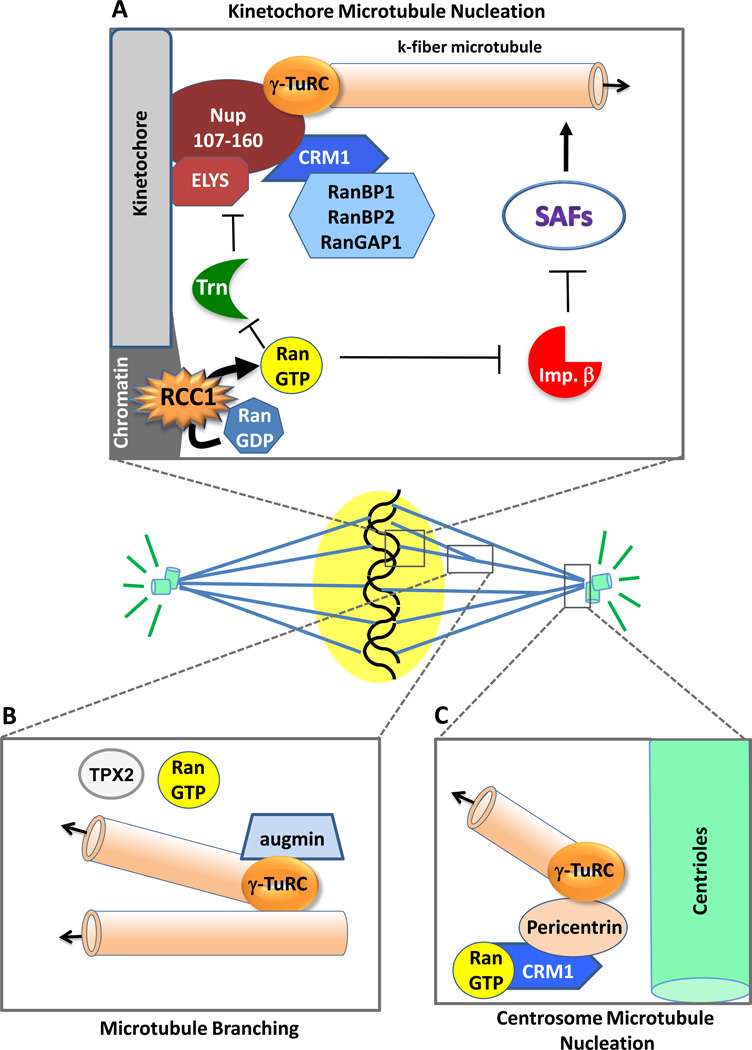
Figure 2. Microtubule nucleation occurs at multiple locales within the spindle and is regulated by RanGTP in each.
(A) In mitosis, microtubules have been shown to grow from γ-TuRC complexes recruited to the kinetochore by the Nup107–160 nucleoporin complex [42]. In humans, the export receptor Crm1 is also present at the kinetochore with its binding partners RanGap1, RanBP1, and RanBP2. The RanGEF RCC1 is present on mitotic chromosomes and, as described in Legend 1B, produces a gradient (“cloud”) of RanGTP around the chromosomes. RanGTP frees local SAFs from inhibition by importin beta (multiple SAFs) and transportin (the Nup107/160 complex and Elys SAFs) [9,10,49,52], such that k-fiber microtubules can grow from the kinetochores. (B) A newly discovered branching mechanism acting in spindle microtubule assembly is depicted. Here assembly can occur not only from centrosomes and kinetochores, as has long been known, but also from the sides of existing spindle microtubules. Augmin and γ-TuRC complexes are involved in the initiation of branching; RanGTP and TPX2 are also involved, but their exact mechanistic roles remain unknown [53–55]. (C) Spindle microtubules also initiate strongly from the centrosomes in many instances [35], nucleated by γ-TuRC complexes. Exportin Crm1/RanGTP aids in the recruitment of pericentrin to the centrosome region, which then recruits the γ-TuRC complexes [91].