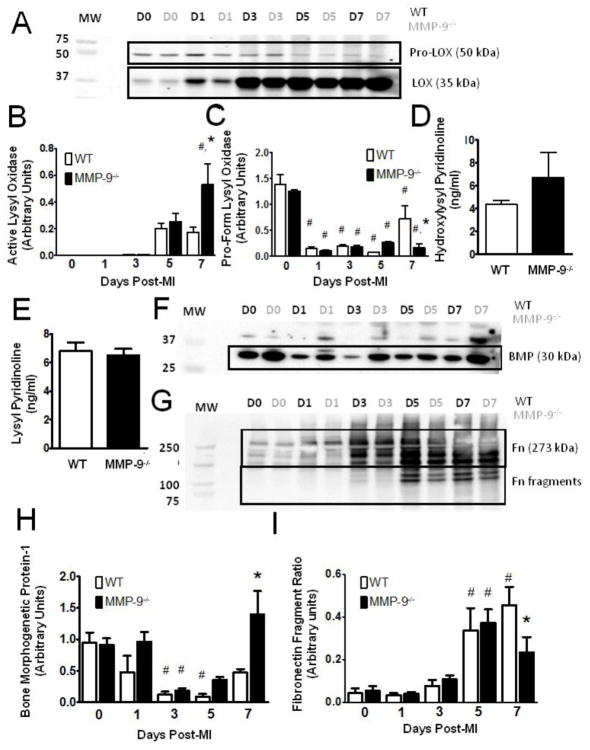
Figure 6. Matrix metalloproteinase-9 (MMP-9) deletion promotes collagen fibril formation and crosslinking.
A. Immunoblot for lysyl oxidase (LOX) showing both inactive and active forms. (D = day, MW = molecular weight) B. Immunoblotting revealed significantly elevated levels of active LOX in the MMP-9−/− group as compared to both the baseline MMP-9−/− group and the WT day 7 MI group. C. The pro-form of LOX was significantly decreased from baseline in all MI groups, but the reduction was attenuated in the absence of MMP-9. D–E. Neither hydroxylysyl pyridinoline or lysyl pyrridinoline content was significantly different between the two groups at day 7 post-MI F–G. Immunoblots for bone morphogenetic protein-1 (BMP) and fibronectin (Fn), respectively. H. Expression of bone morphogenetic protein-1 was significantly reduced from baseline at day 3 and 5 post-MI in WT and at day 3 post-MI in MMP-9−/−. Bone morphogenetic protein-1 expression was significantly elevated in the MMP-9−/− group at day 7 compared to WT. H. The ratio of the amounts of 120 and 80 kDa fibronectin fragments to the amount of 273 kDa full-length fibronectin revealed significantly elevated fragmentation of fibronectin at day 5 and 7 in the WT group and at day 5 in the MMP-9−/− group. The fragmentation ratio was significantly reduced in the MMP-9−/− group as compared to WT at day 7. (n=8 per group; #p<0.05 vs. corresponding baseline group, *p<0.05 vs. WT at corresponding day)