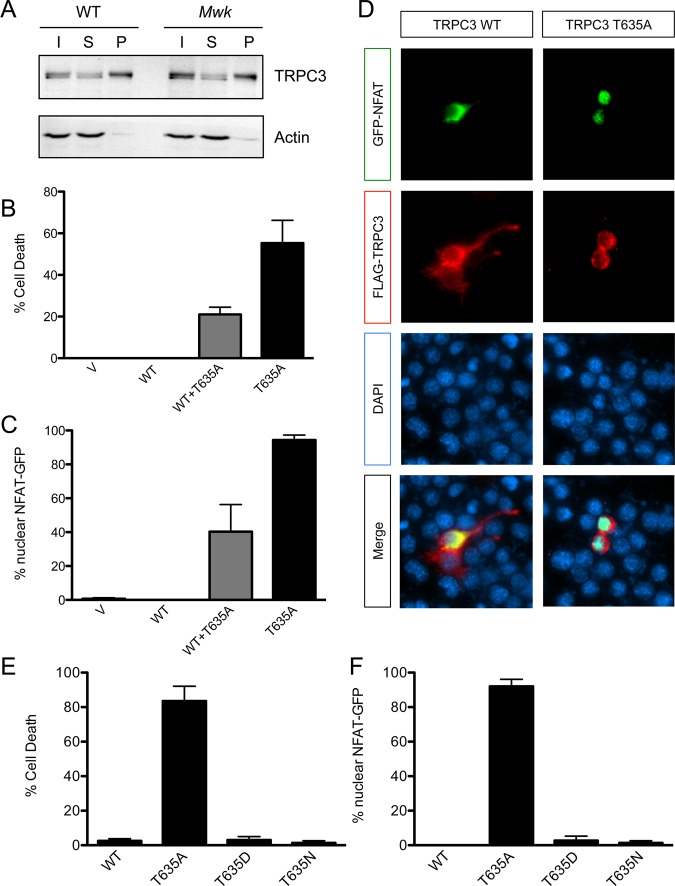
Figure 2.
The gain-of-function Mwk mutation in TRPC3 causes increased cell death and calcium signaling in neuronal cells. (A) Equal amounts of TRPC3 are expressed at the cell surface in the mouse cerebellum. Biotinylated cerebellar slice cultures from wild-type (WT) and Mwk mice were lysed and subjected to pull-down experiments using streptavidin beads, followed by immunoblotting for TRPC3 and actin. Abbreviations: I, input; S, supernatant; B, pellet (biotinylated fraction). (B) Overexpression of Mwk (T635A) but not wild-type (WT) TRPC3 significantly induced cell death in mouse Neuro-2a cells (mean ± SEM; n = 3; ANOVA followed by Bonferroni’s post hoc test; p < 0.005). V denotes vector control. (C and D) Overexpression of Mwk (T635A) but not wild-type (WT) TRPC3 causes significant nuclear localization of co-expressed GFP-tagged NFAT (mean ± SEM; n = 3; ANOVA followed by Bonferroni’s post hoc test; p < 0.001). Cells were fixed 24 h after transfection and subjected to indirect immunofluorescence using antibodies against FLAG, GFP, and the DNA dye DAPI. Cells transfected with mutant TRPC3 have a more rounded appearance because of their impending cell death. (E) The in vitro gain-of-function phenotype of Mwk TRPC3 is not likely to be mediated by phosphorylation. Overexpression of the phospho-mimic T635D mutation but also of the control T635N mutation did not induce cell death in mouse Neuro-2a cells (mean ± SEM; n = 3; ANOVA followed by Bonferroni’s post hoc test). (F) Overexpression of the phospho-mimic T635D mutation but also of the control T635N mutation did not cause significant nuclear translocation of GFP-NFAT (mean ± SEM; n = 3; ANOVA followed by Bonferroni’s post hoc test).