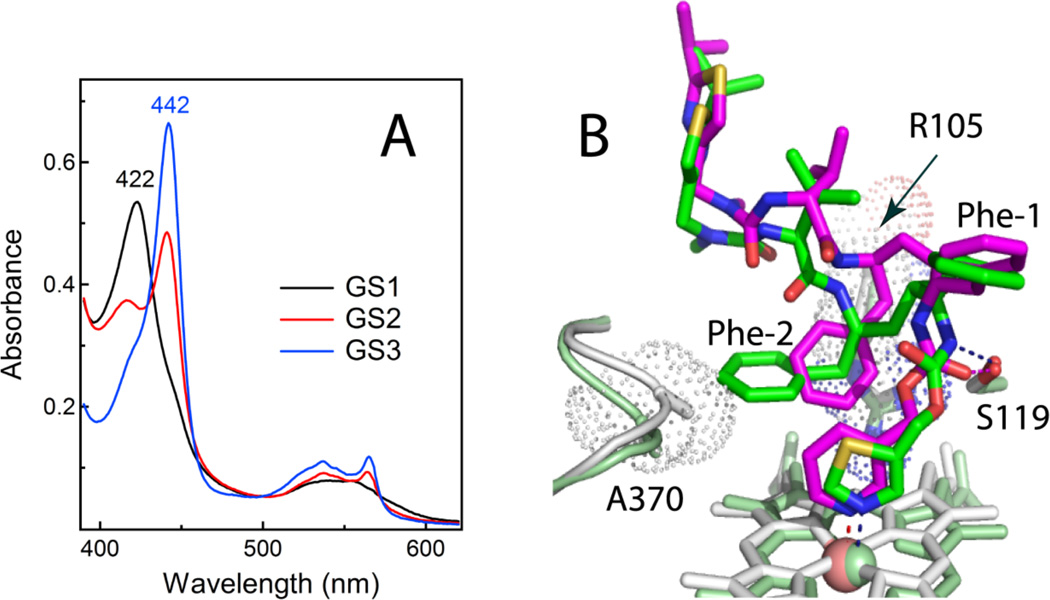
Figure 6.
A, Absorbance spectra of the ferrous GS1-, GS2- and GS3-bound CYP3A4 (black, red and blue, respectively). B, Superposition of the ritonavir- and GS3-bound CYP3A4 structures (green/pale green and magenta/gray, respectively). GS3 has a more flexible backbone and places Phe-2 between the heme-ligating pyridine and the Arg105 guanidinium groups, thereby promoting π-π and cation-π interactions and preventing steric hindrance with the 369–371 peptide. Another notable difference with ritonavir is a peptide bond flip, due to which the GS3 carbonyl oxygen rather than amide nitrogen forms a hydrogen bond with Ser119 (shown as dotted lines).