Abstract
Background
Bendamustine is a bifunctional alkylating agent with unique properties that distinguish it from other agents in its class. Bendamustine is used as monotherapy or in combination with other agents to treat patients with non-Hodgkin lymphoma (nhl) and chronic lymphocytic leukemia (cll).
Methods
The prospective interventional open-label bend-act trial evaluated bendamustine in patients with rituximab-refractory indolent nhl (inhl) and previously untreated cll. Study objectives were to assess the safety and tolerability of bendamustine monotherapy and to provide patients with access to bendamustine before Health Canada approval. The study aimed to enrol up to 100 patients. All patients with inhl received an intravenous dose of bendamustine 120 mg/m2 over 60 minutes on days 1 and 2 for up to eight 21- or 28-day treatment cycles. All patients with cll received an intravenous dose of bendamustine 100 mg/m2 over 30 minutes on days 1 and 2 for up to six 28-day treatment cycles.
Results
Of 90 patients treated on study (16 with cll and 74 with inhl), 35 completed the study (4 with cll and 31 with inhl). The most common treatment-emergent adverse events (teaes) were nausea (70%), fatigue (57%), vomiting (40%), and diarrhea (33%)—mostly grades 1 and 2. Ondansetron was the most common supportive medication used in the patients (63.5% of those with inhl and 68.8% of those with cll). Neutropenia (32%), anemia (23%), and thrombocytopenia (21%) were the most frequent hematologic teaes, with neutropenia being the most common grade 3 or 4 teae leading to dose modification. Dose delays occurred in 28 patients (31.3%) because of grade 3 or 4 teaes, with a higher incidence of dose delays being observed in inhl patients on the 21-day treatment cycle than in those on the 28-day treatment cycle (50.0% vs. 24.1%). During the study, 33 patients (36.7%) experienced at least 1 serious adverse event, and 4 deaths were reported (all in patients with inhl).
Conclusions
The type and frequency of the teaes reported accorded with observations in earlier clinical trials and post-marketing experiences, thus confirming the acceptable and manageable safety profile of bendamustine.
Keywords: Clinical trials, indolent non-Hodgkin lymphoma, chronic lymphocytic leukemia, bendamustine, open-label, expanded-access, safety
BACKGROUND
In Canada, non-Hodgkin lymphoma (nhl) was estimated to account for 4.2% of all new cancer cases in 2014, with an incidence rate exceeding 8000 new cases annually1. Collectively, the indolent B-cell lymphomas account for approximately 40% of all nhls2, and the indolent nhls (inhls) come in various histologic subtypes: follicular lymphoma, marginal zone lymphoma, lymphoplasmacytic lymphoma, and small lymphocytic lymphoma. Follicular lymphoma accounts for approximately one third of nhl cases and is the most common form of inhl3. The median age at diagnosis for patients with follicular lymphoma is 60–65 years, and the incidence of this subtype increases with age4.
For the past several years, the standard initial treatment for patients with inhl included chemo-immunotherapy with either r-chop (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) or r-cvp (rituximab, cyclophosphamide, vincristine, prednisone), followed by a 2-year course of rituximab maintenance5. Although this rituximab-based approach significantly improved the survival rate in patients with inhl, the disease remains incurable, and patients eventually relapse5–8. Additionally, because most patients with inhl are initially treated with a rituximab-containing regimen, they can become refractory to rituximab, after which subsequent treatment options are limited. The radio-immunotherapies, 90Y–ibritumomab tiuxetan and 131I–tositumomab, are treatment options for patients with rituximab-refractory disease, but they are rarely used9–11. Both agents require that patients have less than 25% bone marrow involvement by lymphoma infiltration, an absolute neutrophil count greater than 1500/mm3, and a platelet count greater than 100,000/mm3. Not only do those therapies have strict eligibility criteria, but they are also complex to administer and their adverse event profiles have limited patient and physician acceptance. Moreover, in August 2013, the manufacturer of 131I–tositumomab announced via a press release that they would discontinue the manufacture and sale of that agent on 20 February 201412. Consequently, new therapies for relapsed rituximab-refractory inhl remain an unmet medical need.
Chronic lymphocytic leukemia (cll) is the most common leukemia among adults in the Western world, accounting for approximately 11% of all hematologic cancers13. At diagnosis, the median age of patients is 72 years, and 75% are 65 years of age or older14. In addition, the average patient more than 65 years of age has at least three other health conditions, and 44% have impaired kidney function14–16. The most effective initial treatment regimen for medically fit cll patients is fludarabine–cyclophosphamide–rituximab (fcr), but that regimen is associated with significant toxicities17, making it suitable for only a subset of cll patients—typically those who are young and very fit and who have few comorbidities. Because most patients with cll are older and have other comorbidities, they are not eligible for treatment with fcr. Consequently, improved first-line treatment options are required for those patients.
One potential drug candidate for the treatment of cll and inhl is bendamustine. Bendamustine is a cytotoxic compound that was synthesized as a hybrid molecule intended to combine the activities of the purine antimetabolite benzimidazole with the alkylating properties of the bifunctional nitrogen mustard mechlorethamine18. Although the exact mechanism of action and the contribution of the antimetabolite properties are still not well defined, bendamustine is known to induce dna damage with inter-and intra-strand breaks in a more extensive and durable way than is seen with other alkylators such as melphalan, carmustine, and cyclophosphamide18. Bendamustine induces cell death by activating a stress response to dna damage, with subsequent apoptosis, and by inhibiting several mitotic checkpoints19. Interestingly, it can also use non-apoptotic mechanisms to trigger cell death by inducing mitotic catastrophe in a p53-independent manner. Most importantly, bendamustine exhibits only partial cross- resistance with other alkylators, making it an excellent option for front-line treatment and for relapsed patients or patients who are refractory to prior therapies5,18–20.
After bendamustine was approved in the United States in 2008 and in Europe in 2010, Health Canada approved bendamustine (Treanda: Lundbeck, Copenhagen, Denmark) in August 2012 for the treatment of relapsed inhl21–23. The Health Canada approval was based on a single-arm study in 100 patients with relapsed B-cell inhl that had progressed during or within 6 months of treatment with rituximab or a rituximab-containing regimen24. The overall response rate (orr) was 75%, and the median duration of response was 9.2 months. In the trial, bendamustine was well tolerated, with 60% of patients receiving at least 6 cycles of treatment. The most frequently reported adverse events were blood and lymphatic disorders (anemia, thrombocytopenia, neutropenia) and gastrointestinal disorders (nausea, diarrhea, vomiting).
Bendamustine in combination with rituximab was also evaluated in the first-line management of inhl by Rummel et al.25 in the stil1 study. In that study, the bendamustine–rituximab combination was compared with r-chop and demonstrated prolonged progression-free survival (pfs) and fewer hematologic toxicities.
In August 2012, Health Canada also approved bendamustine for the first-line treatment of symptomatic cll21–23. That approval was based on the results from an open-label randomized controlled multicentre study comparing bendamustine with chlorambucil26. The study was conducted in 319 patients with previously untreated cll Binet stage B or C (Rai stage ii–iv) requiring treatment. Compared with patients receiving chlorambucil, those receiving bendamustine experienced a higher orr (68% vs. 31% with chlorambucil, p < 0.0001) and a longer median pfs (21.6 months vs. 8.3 months, p < 0.0001). For patients treated with bendamustine, the most frequently reported adverse events were neutropenia, thrombocytopenia, and anemia26.
Purpose
The primary objective of the bend-act study was to evaluate the safety of bendamustine in Canadian patients with inhl who had progressed during or after treatment with a rituximab-based regimen or in patients with previously untreated cll. Although the efficacy and safety of bendamustine had been examined in several clinical trials, bend-act was the first study to evaluate its safety in a Canadian patient population. The bend-act study was initiated in December 2011, when bendamustine was not yet available to Canadian patients; consequently, the study also provided hematologists and patients with access to bendamustine during that period.
METHODS
The prospective interventional open-label phase iiib bend-act study was conducted at 16 centres (18 sites) across Canada between 5 March 2012 and 4 June 2013. The study was designed and conducted in accordance with the principles of the Declaration of Helsinki and in compliance with its protocol, the principles of Good Clinical Practice, and applicable regulatory requirements. This study was approved by the research ethics board at each site, and eligible patients provided written informed consent before participating. The safety and tolerability of bendamustine were the primary study endpoints. Safety endpoints included the incidence of treatment-emergent adverse events (teaes), serious adverse events (saes), death, dose delays, and dose reductions.
Study Population
The 99 patients screened for enrolment had either inhl that had progressed during or shortly after treatment with a rituximab-based regimen or previously untreated cll (World Health Organization Criteria27). Patients 18 years of age or older were eligible to enrol, with no upper age limit. To be included in the study, patients also required an Eastern Cooperative Oncology Group performance status in the 0–2 range28 and adequate hepatic and renal function (≤2.5 times the upper limit of the normal laboratory range for aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase; ≤1.5 times the upper limit of the normal laboratory range for total bilirubin; and a creatinine clearance of 40 mL/min or more). Patients were excluded if they had active transformed lymphoma or any history of central nervous system or leptomeningeal lymphoma; if they had an active malignancy other than the target cancer within the preceding 5 years; or if they were positive for hiv. Also excluded from the trial were patients with a clinically significant unstable illness that would make it unsafe for them to receive bendamustine and those who had previously received high-dose chemotherapy with allogeneic stem-cell support.
For patients with inhl, additional eligibility criteria included a biopsy-confirmed diagnosis of indolent B-cell nhl documented as relapsed or refractory after rituximab-based therapy. In addition, patients with inhl were required to have adequate hematologic function (hemoglobin ≥ 100 g/L, absolute neutrophil count ≥ 1.5×109/L, platelet count ≥ 100×109/L) unless abnormalities were related to lymphoma involvement of the bone marrow or hypersplenism caused by lymphoma. Patients were required to have one of the following B-cell lymphoma histologies: follicular lymphoma grade 1, 2, or 3A; marginal zone lymphoma; lymphoplasmacytic lymphoma; or small lymphocytic lymphoma. Patients also were required to have a clinical need for treatment based on evaluation by the treating physician.
For patients with cll, additional eligibility criteria included having previously confirmed and untreated symptomatic B-cell cll Binet stage B or C29 (or Rai stage ii–iv30) requiring treatment, as defined by one of these criteria: hematopoietic insufficiency with non-hemolysis-induced hemoglobin (<10 g/dL), thrombocytopenia (<100×109/L), B symptoms, rapidly progressive disease, or risk of organ complications from bulky lymphoma.
Treatment
All patients with inhl received an intravenous dose of bendamustine 120 mg/m2 over 60 minutes on days 1 and 2 every 21 or 28 days, at the discretion of the treating physician, for up to 8 cycles. All patients with cll received an intravenous dose of bendamustine 100 mg/m2 over 30 minutes on days 1 and 2 every 28 days, for up to 6 cycles of treatment.
Dosing and infusion times for inhl and cll both accorded with the U.S. prescribing information for Treanda31 given that bendamustine had not yet been approved in Canada. The dosing and infusion times in the current Canadian product monograph are consistent with the U.S. prescribing information32. Any medications that were used either before the first dose of bendamustine or to treat teaes or disease-related symptoms were supported in the study and were administered according to the institution’s standard of care or at the discretion of the investigator.
Dose Modifications
In the event of toxicities related to bendamustine treatment, the dose of bendamustine could be delayed, or reduced, or both. All dose modifications (that is, delays and reductions) were made according to the U.S. prescribing information31 given that bendamustine had not yet been approved in Canada.
Dose Delays
In patients with inhl or cll, treatment with bendamustine was delayed in the event of grade 4 hematologic toxicity or clinically significant grade 2 or greater nonhematologic toxicity.
Once hematologic toxicity had recovered to grade 1 or less or blood counts had improved (absolute neutrophil count ≥ 1×109/L, platelets ≥ 75×109/L), or both, bendamustine was reinitiated at the discretion of the treating physician.
Dose Reductions
In inhl patients with grade 4 hematologic toxicity, the dose was reduced to 90 mg/m2 on days 1 and 2 of each cycle, and further reduced to 60 mg/m2 if grade 4 hematologic toxicity recurred. For clinically significant grade 3 or greater nonhematologic toxicity, the dose was reduced to 90 mg/m2 on days 1 and 2 of each cycle, and further reduced to 60 mg/m2 if grade 3 toxicity recurred. If clinically significant grade 3 nonhematologic toxicity was not resolved at 60 mg/m2, the patient was withdrawn from the study.
In cll patients with grade 3 or greater hematologic toxicity or clinically significant grade 3 or greater nonhematologic toxicity, the dose was reduced to 50 mg/m2 on days 1 and 2 of each cycle. If grade 3 or greater hematologic toxicity recurred, the dose was reduced to 25 mg/m2 on days 1 and 2 of each cycle, and if toxicity did not resolve at 25 mg/m2, the patient was withdrawn from the study.
Safety Assessment
Safety endpoints included the incidence of teaes, saes, deaths, use of supportive medications (any concomitant medication given before or after the first dose of bendamustine), and grade 3 or 4 teaes resulting in dose delay or reduction. Additionally, the incidence of saes and grades 3 and 4 toxicities resulting in dose delays or reductions were stratified by cycle length for inhl patients and by age for cll patients. All enrolled patients who took at least 1 dose of bendamustine were evaluable for safety. The study was considered completed once the safety follow-up visit (at 6 weeks after the last dose of bendamustine, or at 2 weeks after the last dose if a patient was removed from the study prematurely) was finished.
Before administration of bendamustine on days 1 and 2 of each treatment cycle, patients underwent clinical laboratory tests (hematologic, liver, kidney, electrolytes, serology, and nutrition), a physical examination (at minimum, examination of appearance, extremities, skin, head, neck, eyes, ears, nose, throat, lungs, chest, heart, abdomen, genitourinary system, and musculoskeletal system), and assessment of vital signs and Eastern Cooperative Oncology Group performance status. Adverse events and supportive medications were recorded throughout the study at the investigator’s discretion and as part of routine care. Toxicities were graded according to the Common Terminology Criteria for Adverse Events (version 4.0)33 from the U.S. National Cancer Institute’s Cancer Therapy Evaluation Program. Serious adverse events were defined as any adverse event that resulted in death, was life-threatening, required inpatient hospitalization or prolongation of existing hospitalization, resulted in persistent or significant disability or incapacity, resulted in a congenital anomaly or birth defect, or was medically important.
Statistical Analysis
All safety analyses included any enrolled patient who received at least 1 dose of bendamustine. Data from clinical assessments were summarized using descriptive techniques and are presented for all patients and by disease subtype (inhl vs. cll). The principal statistical software used in the analysis was SAS (version 9.2: SAS Institute, Cary, NC, U.S.A.). Although no formal power calculations were performed, the sample size of up to 100 patients was considered sufficient for fulfilling the study objectives.
RESULTS
Patient Characteristics
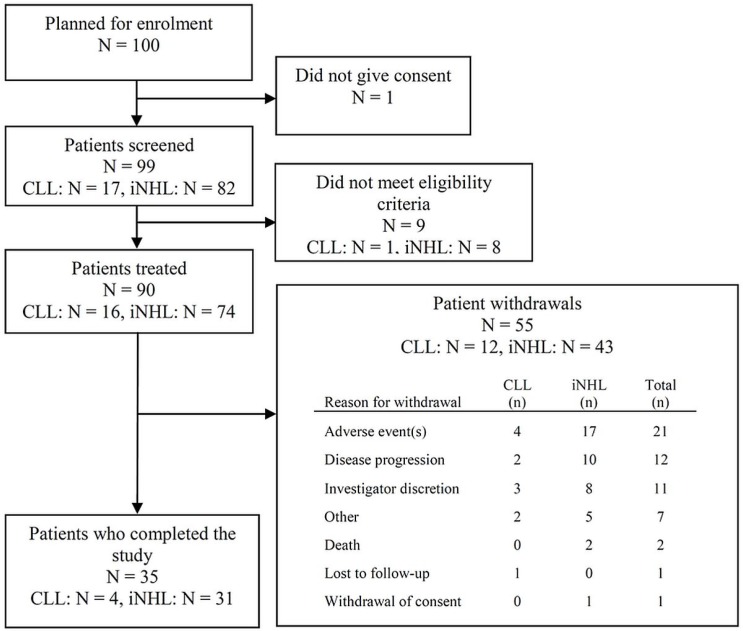
Of 99 patients screened for the study, 90 received at least 1 dose of bendamustine (Figure 1). Table i summarizes the characteristics of the study population.
FIGURE 1.
Overview of the BEND-ACT patient population. CLL = chronic lymphocytic leukemia; iNHL = indolent non-Hodgkin lymphoma.
TABLE I.
Baseline characteristics of the study population
| Characteristic | Patient group | ||
|---|---|---|---|
|
| |||
| CLL | iNHL | Overall | |
| Patients (n) | 16 | 74 | 90 |
| Age (years) | |||
| Mean±SD | 69.3±7.12 | 63.1±10.84 | 64.2±10.51 |
| Range | 55–85 | 40–90 | 40–90 |
| Age group [n (%)] | |||
| <70 Years | 9 (56.3) | 52 (70.3) | 61 (67.8) |
| ≥70 Years | 7 (43.8) | 22 (29.7) | 29 (32.2) |
| Sex [n (%)] | |||
| Women | 9 (56.3) | 35 (47.3) | 44 (48.9) |
| Men | 7 (43.8) | 39 (52.7) | 46 (51.1) |
| Race [n (%)] | |||
| White | 15 (93.8) | 66 (89.2) | 81 (90.0) |
| Black | 0 | 2 (2.7) | 2 (2.2) |
| Asian | 0 | 1 (1.4) | 1 (1.1) |
| Other | 1 (6.3) | 5 (6.8) | 6 (6.7) |
| ECOG PS [n (%)] | |||
| 0 | 9 (56.3) | 38 (51.4) | 47 (52.2) |
| 1 | 6 (37.5) | 30 (40.5) | 36 (40.0) |
| 2 | 1 (6.3) | 6 (8.1) | 7 (7.8) |
| Binet stage [n (%)] | |||
| B | 3 (18.8) | NA | 3 (18.8) |
| C | 2 (12.5) | NA | 2 (12.5) |
| Rai stage [n (%)] | |||
| II–IV | 11 (68.8) | NA | 11 (68.8) |
| iNHL type [n (%)] | |||
| Follicular | NA | 42 (56.8) | 42 (56.8) |
| Lymphoplasmacytic | NA | 12 (16.2) | 12 (16.2) |
| Marginal zone | NA | 10 (13.5) | 10 (13.5) |
| Small lymphocytic | NA | 10 (13.5) | 10 (13.5) |
CLL = chronic lymphocytic leukemia; iNHL = indolent non-Hodgkin lymphoma; SD = standard deviation; ECOG PS = Eastern Cooperative Oncology Group performance status; NA = not applicable.
Of the 90 patients analyzed, 74 had been diagnosed with inhl, and 16, with cll. Of the inhl patients, 42 (56.8%) had follicular lymphoma, 12 (16.2%) had lymphoplasmacytic lymphoma, 10 (13.5%) had marginal zone lymphoma, and 10 (13.5%) had small lymphocytic lymphoma. The mean age of the patients overall was 64.2 years: 63.1 years for the inhl patients, and 69.3 years for the cll patients (Table i). Compared with the inhl patient population, the cll population not only had a greater mean age, but also a larger percentage of patients 70 years of age and older (43.8% cll vs. 29.7% inhl).
Patient Disposition
Of the 90 patients treated with bendamustine, 35 (38.9%) completed the study, including 4 patients with cll (25.0%) and 31 patients with inhl (41.9%). Of the 90 patients, 55 (61.1%) left the study early, with adverse events being the most common reason for discontinuation (21 of 90, 23.3%; Figure 1).
iNHL Patients
The mean time on bendamustine treatment for patients with inhl was 137.3 days (range: 28–224 days), and the mean daily dose received per treatment cycle was 208.0 mg (range: 41.3–300.0 mg). Patients were assigned, at the discretion of the physician, to a 21- or 28-day treatment cycle. Overall, 14 patients (18.9%) were treated only on the 21-day treatment cycle, 57 patients (77.0%) were treated only on the 28-day treatment cycle, and 3 patients (4.1%) were treated on both the 21-day and 28-day treatment cycles. Of the 74 patients, 41 (55.4%) started 6 cycles of bendamustine. Interestingly, 20 patients (27.0%) started 7 cycles and 18 patients (24.3%) started 8 cycles of bendamustine treatment.
CLL Patients
For patients with cll, the mean time on bendamustine treatment was 91 days (range: 28–168 days), and the mean daily dose received per treatment cycle was 161.8 mg (range: 47.0–229.0 mg). Of these 16 patients, 4 started the planned 6 cycles, and 10 (62.5%) started at least 3 cycles of bendamustine.
Supportive Medication
For the overall population, supportive medications, including medications started before or after the first dose of bendamustine, were reported for 87 patients (96.7%, Table ii). The most frequently reported supportive medications were ondansetron (n = 58, 64.4%), dexamethasone (n = 37, 41.1%), and prochlorperazine (n = 29, 32.2%). Blood or transfusion-related products were also used as supportive therapy: concentrated red blood cells (n = 13, 14.4%), red blood cells (n = 5, 5.6%), epoetin alfa (n = 4, 4.4%), platelets (n = 4, 4.4%), whole blood (n = 2, 2.2%), and darbepoetin alfa (n = 2, 2.2%).
TABLE II.
Summary of frequent supportive therapies
| Therapy | Patient group [n (%)] | ||
|---|---|---|---|
|
| |||
| CLL (n=16) | iNHL (n=74) | Overall (n=90) | |
| Any | 15 (93.8) | 72 (97.3) | 87 (96.7) |
| Ondansetron | 11 (68.8) | 47 (63.5) | 58 (64.4) |
| Dexamethasone | 4 (25.0) | 33 (44.6) | 37 (41.1) |
| Prochlorperazine | 6 (37.5) | 23 (31.1) | 29 (32.2) |
| Acetaminophen | 6 (37.5) | 19 (25.7) | 25 (27.8) |
| Filgrastim | 5 (31.3) | 20 (27.0) | 25 (27.8) |
| Allopurinol | 11 (68.8) | 11 (14.9) | 22 (24.4) |
| GM-CSF | 0 (0) | 2 (2.7) | 2 (2.2) |
| Blood-related therapy | |||
| Concentrated RBCs | 1 (6.3) | 12 (16.2) | 13 (14.4) |
| RBCs | 1 (6.3) | 4 (5.4) | 5 (5.6) |
| Epoetin alfa | 2 (12.5) | 2 (2.7) | 4 (4.4) |
| Platelets | 1 (6.3) | 3 (4.1) | 4 (4.4) |
| Whole blood | 1 (6.3) | 1 (1.4) | 2 (2.2) |
| Darbepoetin alfa | 0 (0) | 2 (2.7) | 2 (2.2) |
CLL = chronic lymphocytic leukemia; GM-CSF = granulocyte-macrophage colony-stimulating factor; iNHL = indolent non-Hodgkin lymphoma; RBCs = red blood cells.
iNHL Patients
Use of supportive medications was reported in 72 of the patients with inhl (97.3%). The most frequently reported medication in those patients was ondansetron (n = 47, 63.5%). Other medications used in this patient population included dexamethasone (n = 33, 44.6%) and prochlorperazine (n = 23, 31.1%). Anti-infectives, Bactrim [sulfamethoxazole–trimethoprim (Hoffmann–La Roche, Basel, Switzerland)], and quinolones were used as supportive care in, respectively, 39 (52.7%), 6 (8.1%), and 15 (20.3%) patients.
CLL Patients
Ondansetron and allopurinol were the most commonly used supportive medications in patients with cll (11 patients each, 68.8%). Prochlorperazine (n = 6, 37.5%), acetaminophen (n = 6, 37.5%), and filgrastim (n = 5, 31.3%) were also frequently used in these patients. Anti-infectives, Bactrim, and quinolones were used as supportive care in, respectively, 11 (68.8%), 2 (12.5%), and 4 (25.0%) patients.
Adverse Events
All patients treated with at least 1 dose of bendamustine reported 1 or more teaes.
Most patients with inhl (n = 68, 91.9%) and all patients with cll (n = 16, 100%) initially experienced a teae or a worsened teae during cycle 1. No increase in the frequency of teaes or worsening of existing teaes was observed with the number of subsequent bendamustine cycles. The most commonly reported teaes for the population overall were nausea (70.0%), fatigue (56.7%), vomiting (40.0%), and diarrhea (33.3%); most were grade 1 or 2 (Table iii). The incidences of various teaes were similar in the inhl and cll populations (<20% difference), except for dysgeusia, which was reported only by inhl patients (n = 17, 23.0%). In general, patients more commonly experienced teaes of grade 2 (31.3% in cll patients and 23.0% in inhl patients) or grade 3 (37.5% in cll patients and 48.6% in inhl patients). Overall, 60 patients (66.7%) experienced at least 1 grade 3 or 4 teae (inhl: 67.5%; cll: 62.5%). In inhl patients, the most common grade 3 or 4 teaes were blood and lymphatic system disorders (n = 30, 40.5%), including neutropenia (n = 20, 27.0%), anemia (n = 12, 16.2%), and thrombocytopenia (n = 6, 8.1%).
TABLE III.
Treatment-emergent adverse events occurring in ten or more patients
| Event | Patient group [n (%)] | ||
|---|---|---|---|
|
| |||
| CLL (n=16) | iNHL (n=74) | Overall (n=90) | |
| Any event | 16 (100.0) | 74 (100.0) | 90 (100.0) |
| Nausea | 12 (75.0) | 51 (68.9) | 63 (70.0) |
| Fatigue | 8 (50.0) | 43 (58.1) | 51 (56.7) |
| Vomiting | 9 (56.3) | 27 (36.5) | 36 (40.0) |
| Diarrhea | 9 (56.3) | 21 (28.4) | 30 (33.3) |
| Neutropenia | 5 (31.3) | 24 (32.4) | 29 (32.2) |
| Constipation | 5 (31.3) | 23 (31.1) | 28 (31.1) |
| Pyrexia | 6 (37.5) | 21 (28.4) | 27 (30.0) |
| Decreased appetite | 4 (25.0) | 20 (27.0) | 24 (26.7) |
| Headache | 5 (31.3) | 17 (23.0) | 22 (24.4) |
| Anemia | 4 (25.0) | 17 (23.0) | 21 (23.3) |
| Thrombocytopenia | 5 (31.3) | 14 (18.9) | 19 (21.1) |
| Dysgeusia | 0 | 17 (23.0) | 17 (18.9) |
| Cough | 3 (18.8) | 13 (17.6) | 16 (17.8) |
| Dyspepsia | 1 (6.3) | 15 (20.3) | 16 (17.8) |
| Dizziness | 2 (12.5) | 13 (17.6) | 15 (16.7) |
| Chills | 3 (18.8) | 11 (14.9) | 14 (15.6) |
| Edema peripheral | 2 (12.5) | 12 (16.2) | 14 (15.6) |
| Decreased platelet count | 2 (12.5) | 11 (14.9) | 13 (14.4) |
| Dry mouth | 2 (12.5) | 10 (13.5) | 12 (13.3) |
| Dyspnea | 3 (18.8) | 9 (12.2) | 12 (13.3) |
| Decreased neutrophil count | 4 (25.0) | 7 (9.5) | 11 (12.2) |
| Decreased weight | 2 (12.5) | 9 (12.2) | 11 (12.2) |
| Oropharyngeal pain | 2 (12.5) | 8 (10.8) | 10 (11.1) |
| Rash | 2 (12.5) | 8 (10.8) | 10 (11.1) |
CLL = chronic lymphocytic leukemia; iNHL = indolent non-Hodgkin lymphoma.
During the study, 33 patients (36.7%) experienced at least 1 sae (Table iv). The most frequent saes were pyrexia (n = 9, 10.0%), febrile neutropenia (n = 5, 5.6%), pneumonia (n = 3, 3.3%), and acute renal failure (n = 3, 3.3%). Only inhl patients experienced the saes of pneumonia (4.1%), abdominal pain (2.7%), nausea (2.7%), Pneumocystis jiroveci pneumonia (2.7%), and syncope (2.6%). Only cll patients experienced the sae of tumour lysis syndrome [tls (12.5%)]. In the inhl population, the incidence of saes was compared for patients treated on the 21- and 28-day treatment cycles. Of patients on the 21-day treatment cycle, 25.0% (4 of 16) reported saes; of patients on the 28-day cycle, 39.7% (23 of 58) reported saes. Of the 6 cll patients reporting an sae, 4 were 70 years of age or older, 2 of whom experienced tls.
TABLE IV.
Serious adverse events occurring in two or more patients
| Event | Patient group [n(%)] | ||
|---|---|---|---|
|
| |||
| CLL (n=16) | iNHL (n=74) | Overall (n=90) | |
| Any event | 6 (37.5) | 27 (36.5) | 33 (36.7) |
| Pyrexia | 3 (18.8) | 6 (8.1) | 9 (10.0) |
| Febrile neutropenia | 1 (6.3) | 4 (5.4) | 5 (5.6) |
| Pneumonia | 0 | 3 (4.1) | 3 (3.3) |
| Acute renal failure | 1 (6.3) | 2 (2.7) | 3 (3.3) |
| Abdominal pain | 0 | 2 (2.7) | 2 (2.2) |
| Hypotension | 1 (6.3) | 1 (1.4) | 2 (2.2) |
| Nausea | 0 | 2 (2.7) | 2 (2.2) |
| Pneumocystis jiroveci pneumonia | 0 | 2 (2.7) | 2 (2.2) |
| Syncope | 0 | 2 (2.7) | 2 (2.2) |
| Tumour lysis syndrome | 2 (12.5) | 0 | 2 (2.2) |
| Vomiting | 0 | 2 (2.7) | 2 (2.2) |
CLL = chronic lymphocytic leukemia; iNHL = indolent non-Hodgkin lymphoma.
In 4 inhl patients, teaes resulted in death. The teaes resulting in death included Pneumocystis jiroveci pneumonia, multiorgan failure, cardiac arrest and respiratory failure, and abdominal pain; only 1 death (from multiorgan failure) was thought to be related to bendamustine treatment. Consequently, mortality in the study group overall was 4.4%.
Dose Modifications
Dose Delays
In the study group overall, dose delays because of grade 3 or 4 teaes occurred in 28 patients (31.1%). The most frequent grade 3 or 4 teaes leading to dose delay were neutropenia (n = 9, 10.0%), decreased neutrophil count (n = 5, 5.6%), anemia (n = 4, 4.4%), and decreased platelet count (n = 4, 4.4%).
In the inhl population, 22 of 74 patients (29.7%) experienced dose delays because of teaes. The most frequently reported teaes causing dose delays (Table v) included neutropenia (n = 8, 10.8%), decreased neutrophil count (n = 4, 5.4%), decreased platelet count (n = 4, 5.4%), and anemia (n = 3, 4.1%). Dose delays resulting from grade 3 nonhematologic toxicities were reported in 7 patients with inhl (9.5%). Dose delays because of grade 3 or 4 teaes occurred more often in inhl patients on the 21-day treatment cycle than in those on the 28-day cycle (50.0% vs. 24.1%). Neutropenia was the only grade 3 or 4 teae resulting in dose delay whose incidence was higher depending on cycle length: it occurred in 4 of 16 patients on 21-day treatment cycles (25.0%), and in 4 of 58 patients on 28-day treatment cycles (6.9%).
TABLE V.
Most frequent grade 3 or 4 treatment-emergent adverse events resulting in dose delay
| Event | iNHL patients [n (%) by cycle length] | CLL patients [n (%) by age] | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| 21 Days (n=16) | 28 Days (n=58) | Overall (n=74) | <70 Years (n=9) | ≥70 Years (n=7) | Overall (n=16) | |
| Any event | 8 (50.0) | 14 (24.1) | 22 (29.7) | 3 (33.3) | 3 (42.9) | 6 (37.5) |
| Neutropenia | 4 (25.0) | 4 (6.9) | 8 (10.8) | 0 | 1 (14.3) | 1 (6.3) |
| Decreased neutrophil count | 0 | 4 (6.9) | 4 (5.4) | 1 (11.1) | 0 | 1 (6.3) |
| Decreased platelet count | 0 | 4 (6.9) | 4 (5.4) | NA | NA | NA |
| Anemia | 1 (6.3) | 2 (3.4) | 3 (4.1) | 1 (11.1) | 0 | 1 (6.3) |
| Infections and infestations | 1 (6.3) | 2 (3.4) | 3 (4.1) | NA | NA | NA |
| Fatigue | 1 (6.3) | 1 (1.7) | 2 (2.7) | NA | NA | NA |
| Hemolysis | NA | NA | NA | 0 | 1 (14.3) | 1 (6.3) |
| Thrombocytopenia | 1 (6.3) | 0 | 1 (1.4) | 0 | 1 (14.3) | 1 (6.3) |
| Papular rash | NA | NA | NA | 1 (11.1) | 0 | 1 (6.3) |
iNHL = indolent non-Hodgkin lymphoma; CLL = chronic lymphocytic leukemia; NA = not applicable.
In the cll population, 6 of 16 patients (37.5%) experienced dose delays because of grade 3 or 4 teaes. The reported grade 3 teaes (Table v) included anemia, hemolysis, neutropenia, papular rash, and thrombocytopenia, each occurring in 1 patient (6.3%). One patient experienced a grade 4 teae (decreased neutrophil count) causing a dose delay. The frequency of dose delays was similar in cll patients less than 70 years of age (3 of 9 patients, 33.3%) and in those 70 years of age and older (3 of 7 patients, 42.9%).
Dose Reductions
Dose reductions because of grade 3 or 4 teaes occurred in 7 patients overall (7.8%). The most frequent grade 3 or 4 teae leading to dose reduction was neutropenia (3 patients, 3.3%). Dose reductions occurred in 6 of the 74 inhl patients (8.1%) because of grade 3 or 4 teaes, including anemia, neutropenia, diarrhea, and fatigue. Neutropenia was the most frequent cause of dose reductions [3 patients (2 classed as grade 4), 4.1%]. The frequency of dose reductions because of teaes was similar in patients on 21-day and 28-day treatment cycles (6.3% and 8.6% respectively). One cll patient (6.3%) in the group 70 years of age and older experienced a teae resulting in a dose reduction (grade 3 tls).
Concomitant Dose Delays and Reductions
Overall, 19 patients treated with bendamustine experienced concomitant dose delay and reduction because of grade 3 or 4 teaes.
Of the inhl patients, 17 (23.0%) experienced grade 3 or 4 teaes leading to dose delay and reduction. The most frequent of those teaes were hematologic: neutropenia (n = 4, 5.4%), decreased platelet count (n = 4, 5.4%), decreased neutrophil count (n = 3, 4.1%), and thrombocytopenia (n = 3, 4.1%). Grade 4 hematologic toxicities causing dose delay and reduction occurred in 4 inhl patients (5.4%). Grade 3 nonhematologic toxicities resulting in concomitant dose delay and reduction were reported for 6 inhl patients (8.1%), the most frequent being fatigue (4 patients, 5.4%). The frequency of concomitant dose delay and reduction because of teaes was similar for both treatment cycle lengths (25.0% for those on a 21-day cycle and 22.4% for those on a 28-day cycle).
In the cll subgroup, 2 of the 16 patients (12.5%) experienced concomitant dose delay and reduction because of grade 3 or 4 teaes. The reported hematologic teaes were grade 4 neutropenia, grade 3 neutropenia, and grade 3 febrile neutropenia (1 patient each, 6.3%). No cll patients experienced grade 3 nonhematologic toxicities leading to concomitant dose delay and reduction.
DISCUSSION
The bend-act study was an open-label expanded-access prospective clinical trial conducted in 16 centres across Canada. Although the safety of bendamustine has been well documented in clinical trials, bend-act is the first study to evaluate the safety of that agent in a Canadian patient population. In addition, the trial provided access to bendamustine before its regulatory approval. The ultimate goal of the study was therefore not only to evaluate the safety of bendamustine, but also to allow health care providers to familiarize themselves with the management of the associated teaes.
In the inhl patient population, the most frequently reported hematologic teaes (Table iii) were neutropenia (32.4%), anemia (23.0%), and thrombocytopenia (18.9%), with grade 3 or 4 events occurring in 27%, 16%, and 8% of patients respectively. Those results are consistent with findings in two phase ii North American clinical trials—Friedberg et al.34 and Kahl et al.24—that also examined bendamustine monotherapy in a rituximab-refractory inhl patient population. Interestingly, compared with incidences in the latter two trials, the incidences of grade 3 or 4 neutropenia and thrombocytopenia were lower in the bend-act trial. The Canadian prescribing information for bendamustine suggests a 21-day treatment cycle for patients with inhl because that regimen was the one used in early clinical trials24,32,34,35. However, the bend-act study allowed a 21- or 28-day treatment cycle for inhl, consistent with the recommendations from a panel of international investigators indicating that a 4-week cycle should be used to reduce hematologic toxicity, dose modifications, and treatment delays36. Although the bend-act trial was not designed to compare outcomes stratified by cycle duration, the reduced number of hematologic toxicities observed in the current study could be a result of most inhl patients being treated on a 28-day cycle11. Interestingly, dose delays occurred in 50.0% of inhl patients on the 21-day treatment cycle and in 24.1% of inhl patients on the 28-day cycle, suggesting that patients had more difficulty tolerating the 21-day cycle. Moreover, the incidence of neutropenia—the only grade 3 or 4 teae resulting in dose delay—was higher in patients on the 21-day cycle (25%) than in those on the 28-day cycle (6.9%). Taken together, those results might provide support for use of the 28-day treatment cycle, which is routine with bendamustine in many treatment centres across Canada for the management of inhl37.
In the present study, the incidences of teaes in the cll patient population also accorded with the safety results reported from the phase iii clinical trial by Knauf et al. comparing bendamustine with chlorambucil in previously untreated cll26. The incidences of hematologic toxicities were similar in both studies (<10% difference); however, increases in nausea (75% vs. 19%), vomiting (56% vs. 16%), diarrhea (56% vs. 10%), and fatigue (50% vs. 9%) of any grade were observed in the bend-act trial. Interestingly, the frequency of dose delays was similar in cll patients less than 70 years of age (3 of 9 patients) and in those 70 years of age and older (3 of 7 patients), which accords with the current Canadian prescribing information for Treanda stating that no clinically significant differences in efficacy and adverse reaction profile were observed between geriatric (≥65 years) and younger patients32. However, caution must be observed in interpreting those results, because the bend-act study included only a very small number of cll patients (n = 16).
The most frequent nonhematologic adverse events observed in the overall bend-act patient population were nausea (70.0%), fatigue (56.7%), vomiting (40.0%), and diarrhea (33.3%), most of which were grade 1 or 2. Nausea, which occurred in 70% of patients overall, was the most frequent nonhematologic adverse event, and accordingly, ondansetron was also the most frequently administered supportive medication (64.4% of patients overall). Other anti-emetic therapies, including dexamethasone and prochlorperazine, were also frequently used. Bendamustine has a moderate emetogenic risk, and consequently, administration of a 5-HT3 antagonist such as ondansetron 1–2 days before, during, and after bendamustine treatment is recommended37,38. To improve the anti-emetic effect and to prevent infusion and skin reactions, dexamethasone can also be given in combination with 5-HT3 antagonists 30 minutes before bendamustine infusion39. In the event of persistent nausea or severe vomiting, administration of aprepitant can be considered. However, it is important to note that aprepitant is reimbursed only for the prevention of nausea and vomiting from highly emetogenic cancer chemotherapy, and consequently, its use in Canada might be limited36,38,39. Acetaminophen, which was used as supportive therapy in 27.8% of patients overall, is also suggested before infusion in patients who have previously experienced grade 1 or 2 infusion reactions (for example, fever, chills, rash)37,38.
The rates of nausea in our study were similar to those previously reported in the relapsed setting. In a study by Kahl et al.24, the rate of all-grade nausea was 77%. Anti-emetic therapy (≤2 doses per cycle) was allowed, but not controlled, in that study. In addition, a study by Friedberg et al.34 gave bendamustine over 30–60 minutes and found a rate of all-grade nausea of 72%.
A paper by Koolwine et al.37 suggested that a shorter bendamustine infusion time might increase peak plasma levels, resulting in more frequent nausea. The authors recommended giving bendamustine to all patients in a 60-minute infusion. However, given that the bend-act study included a small proportion of patients with cll whose infusion time was shorter (n = 16), their experiences are unlikely to have influenced nausea rates. In addition, when stratified by disease type, rates of nausea were similar in the disease subgroups (75% cll vs. 68.9% inhl). It is therefore unlikely that the shorter infusion time in the cll patients significantly affected the rates of nausea observed.
During the study, 33 patients overall (36.7%) experienced at least 1 sae. The most frequent saes (Table iv) were pyrexia (n = 9, 10.0%), febrile neutropenia (n = 5, 5.6%), pneumonia (n = 3, 3.3%), and acute renal failure (n = 3, 3.3%). Tumour lysis syndrome, the second-most frequent sae experienced by cll patients in the bend-act trial, was reported in 2 patients (neither event resulted in death). In patients with cll, allopurinol and ondansetron were administered equally frequently because of the potential risk of tls. Tumour lysis syndrome has previously been reported after bendamustine therapy, particularly in patients with a high tumour burden, although the overall incidence of tls is rare26. Patients at high risk of tls must be identified, and preventive measures (allopurinol initiated before chemotherapy) should be implemented. However, it is important to note that the co-administration of bendamustine and allopurinol can increase the risk of severe skin toxicity (Stevens–Johnson syndrome and toxic epidermal necrolysis) as reported in the Canadian product monograph for Treanda32.
A low overall mortality rate of 4.4% was observed in the bend-act study population. The 4 deaths occurred in the inhl patient population, which is within reasonable limits, considering that the patients were heavily pretreated. Moreover, only 1 death (1%) was considered to be related to bendamustine treatment. In the study by Kahl et al.24, 7 deaths were reported in the inhl patient population (7%), 4 of which were considered to be related to, or most likely related to, bendamustine (4%). However, the Friedberg et al.34 trial did not report any deaths in patients with inhl during bendamustine treatment.
The tolerability of bendamustine was also assessed by recording dose modifications. In the bend-act study, dose modifications occurred in 60% of patients overall. Similarly, Kahl et al.24 reported dose modifications in 68% of patients with inhl. In bend-act, neutropenia was the most frequent grade 3 or 4 teae leading to dose delays and dose reductions in patients with inhl. Consequently, filgrastim or pegfilgrastim was used in 31.1% of the patients treated with bendamustine, similar to their rate of use (38%) in the study by Kahl et al.24. Cancer patients with severe myelosuppression during the first cycle of chemotherapy can be candidates for growth-factor support in subsequent cycles, when maintenance of dose intensity is important36,40. However, it should be noted that, in many provinces, granulocyte colony-stimulating factors cannot be accessed for noncurative therapies such as bendamustine, leaving dose reductions and delays as the only options for managing neutropenia in those provinces.
Although bendamustine was evaluated as monotherapy in the present study, bendamustine in combination with rituximab (br) has also been evaluated in relapsed inhl41. A multicentre randomized phase iii study compared the efficacy and safety of br with that of fludarabine–rituximab for patients with relapsed inhl or mantle cell lymphoma. Recently, updated results from that trial showed significantly prolonged median pfs and median overall survival (os) associated with br (median pfs: 34 months vs. 12 months; p < 0.0001; median os: 110 months vs. 49 months; p = 0.0125). In addition, the orr and complete response rate were significantly higher with br than with fludarabine–rituximab (orr: 83.5% vs. 52.5%; p < 0.0001; complete response: 38.5% vs. 16.2%; p = 0.0004)41. Moreover, there were no significant differences in the rates of nonhematologic and hematologic toxicities between the groups, and the overall incidence of saes was similar for the br and fludarabine–rituximab groups.
Bendamustine in combination with rituximab has also been evaluated in the first-line management of both inhl and cll. From an inhl perspective, results from the stil1 study by Rummel et al.25 indicate that br is not only more effective than the most frequently used first-line option, r-chop, but also better tolerated, with fewer hematologic toxicities. Accordingly, the Alberta and BC Cancer Agency guidelines now recommend 6 courses of br as the preferred first-line treatment for the management of inhl and mantle cell lymphoma42,43. The Lymphoma Canada guidelines for the first-line treatment of follicular lymphoma also recommend br as the preferred regimen44. Updated results after 7 years of follow-up from the stil1 study demonstrated that br has pfs and time-to-next-treatment benefit over r-chop. Although the os for the patients overall was not significantly different, treatment with br was associated with a trend toward survival benefit in the group of patients with indolent lymphomas45.
From a cll perspective, the phase iii cll10 study is currently testing the noninferiority hypothesis with respect to the efficacy and potentially better tolerability of br compared with fcr in the first-line treatment of patients with advanced cll. Results from the final analysis indicate that, compared with br, fcr yields higher complete response rates, more minimal residual disease negativity, and longer pfs. However, a subgroup analysis revealed that there was no significant difference in pfs between br and fcr in patients 65 years of age and older. Moreover, no difference in os was observed between the treatment arms46. From a safety perspective, severe neutropenia was more frequent in patients treated with fcr than with br (87.7% vs. 67.8%, p < 0.001), as was severe infection (39.8% vs. 25.4%, p = 0.001), particularly in elderly patients (48.4% vs. 26.8%, p = 0.001)46. Taken together, those results suggest that fcr remains the standard therapy in very fit cll patients; however, br should be a recommended option in the first-line management of cll patients 65 years of age and older. In fact, based on the cll10 results, Alberta recently updated their guidelines on the management of cll and currently recommends br in the older patient population47.
CONCLUSIONS
The overall safety results from the bend-act study are consistent with those reported in earlier clinical trials and are also aligned with the currently available Canadian product monograph for Treanda. Consequently, the results of the study confirm the acceptable and manageable safety profile of bendamustine in the treatment of patients with inhl and cll. The bend-act study also highlights the use of supportive medications to prevent and manage the adverse events associated with bendamustine treatment. Finally, the safety results and the experience gained from bend-act should increase the confidence of hemato-oncologists using bendamustine-containing regimens in eligible Canadian patients with inhl and cll.
ACKNOWLEDGMENTS
This study was funded by Lundbeck Canada Inc. (Montreal, QC). Lundbeck Canada Inc. thanks all the Canadian investigators who contributed to this trial: Drs. Sarit Assouline, Inès Chamakhi, Neil Chua, Michael Crump, Greg Duek, Caroline Hamm, Jason Hart, James Johnston, Tom Kouroukis, Philip Kuruvilla, Jean-François Larouche, David MacDonald, Carolyn Owen, Laurie Sehn, Chaim Shustik, Julie Stakiw, and Richard H.C. van der Jagt.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: Medical writing support provided by Anna Christofides and Sarah Di Clemente of New Evidence was funded by Lundbeck Canada Inc. MC has received honoraria from Lundbeck Canada Inc.; DAS has received honoraria and travel grants from Lundbeck Canada Inc.; JJ has received consulting fees from Lundbeck Canada Inc.; CTK and LS have received consulting fees and honoraria from Lundbeck Canada Inc.; JFL and DM have received research funding, consulting fees, and honoraria from Lundbeck Canada Inc. SS, EB, PS, and SGD are employees of Lundbeck Canada Inc.
REFERENCES
- 1.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian Cancer Statistics 2014. Toronto, ON: Canadian Cancer Society; 2014. [Available online at: http://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2014-EN.pdf; cited 18 March 2014] [Google Scholar]
- 2.Gil L, Kazmierczak M, Kroll-Balcerzak R, Komarnicki M. Bendamustine-based therapy as first-line treatment for non-Hodgkin lymphoma. Med Oncol. 2014;31:944. doi: 10.1007/s12032-014-0944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedman A. Follicular lymphoma: 2014 update on diagnosis and management. Am J Hematol. 2014;89:429–36. doi: 10.1002/ajh.23674. [DOI] [PubMed] [Google Scholar]
- 4.Ma S. Risk factors of follicular lymphoma. Expert Opin Med Diagn. 2012;6:323–33. doi: 10.1517/17530059.2012.686996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van der Jagt R, Laneuville P, Macdonald D, Stewart D, Christofides A, Sehn LH. A Canadian perspective on bendamustine for the treatment of chronic lymphocytic leukemia and non-Hodgkin lymphoma. Curr Oncol. 2012;19:160–8. doi: 10.3747/co.19.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher RI, LeBlanc M, Press OW, Maloney DG, Unger JM, Miller TP. New treatment options have changed the survival of patients with follicular lymphoma. J Clin Oncol. 2005;23:8447–52. doi: 10.1200/JCO.2005.03.1674. [DOI] [PubMed] [Google Scholar]
- 7.Kenkre VP, Kahl BS. What is the best initial therapy for a patient with symptomatic low-grade follicular lymphoma? Cancer J. 2012;18:383–9. doi: 10.1097/PPO.0b013e31826aed6d. [DOI] [PubMed] [Google Scholar]
- 8.Rueda A, Casanova M, Redondo M, Pérez-Ruiz E, Medina-Pérez A. Has the time to come leave the “watch-and-wait” strategy in newly diagnosed asymptomatic follicular lymphoma patients? BMC Cancer. 2012;12:210. doi: 10.1186/1471-2407-12-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witzig TE, Flinn IW, Gordon LI, et al. Treatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20:3262–9. doi: 10.1200/JCO.2002.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Horning SJ, Younes A, Jain V, et al. Efficacy and safety of tositumomab and iodine-131 tositumomab (Bexxar) in B-cell lymphoma, progressive after rituximab. J Clin Oncol. 2005;23:712–19. doi: 10.1200/JCO.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 11.Cheson BD, Friedberg JW, Kahl BS, Van der Jagt RH, Tremmel L. Bendamustine produces durable responses with an acceptable safety profile in patients with rituximab-refractory indolent non-Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk. 2010;10:452–7. doi: 10.3816/CLML.2010.n.079. [DOI] [PubMed] [Google Scholar]
- 12.GlaxoSmithKline (gsk) GSK to discontinue manufacture and sale of the BEXXAR® Therapeutic Regimen (tositumomab and iodine I 131 tositumomab) [press release] Mississauga, ON: GSK; 2013. [Google Scholar]
- 13.Tadmor T, Polliack A. Optimal management of older patients with chronic lymphocytic leukemia: some facts and principles guiding therapeutic choices. Blood Rev. 2012;26:15–23. doi: 10.1016/j.blre.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Eichhorst B, Goede V, Hallek M. Treatment of elderly patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2009;50:171–8. doi: 10.1080/10428190802688517. [DOI] [PubMed] [Google Scholar]
- 15.Stevens LA, Schmid CH, Greene T, et al. Comparative performance of the ckd Epidemiology Collaboration (ckd-epi) and the Modification of Diet in Renal Disease (mdrd) Study equations for estimating gfr levels above 60 mL/min/1.73 m2. Am J Kidney Dis. 2010;56:486–95. doi: 10.1053/j.ajkd.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thurmes P, Call T, Slager S, et al. Comorbid conditions and survival in unselected, newly diagnosed patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2008;49:49–56. doi: 10.1080/10428190701724785. [DOI] [PubMed] [Google Scholar]
- 17.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–74. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 18.Tageja N. Bendamustine: safety and efficacy in the management of indolent non-Hodgkins lymphoma. Clin Med Insights Oncol. 2011;5:145–56. doi: 10.4137/CMO.S6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leoni LM, Bailey B, Reifert J, et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res. 2008;14:309–17. doi: 10.1158/1078-0432.CCR-07-1061. [DOI] [PubMed] [Google Scholar]
- 20.Leoni LM. Bendamustine: rescue of an effective antineoplastic agent from the mid-twentieth century. Semin Hematol. 2011;48(suppl 1):S4–11. doi: 10.1053/j.seminhematol.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Lundbeck receives approval from Health Canada for Treanda® (bendamustine hydrochloride for injection) to treat patients with relapsed indolent B-cell non-Hodgkin’s lymphoma and chronic lymphocytic leukemia [press release] Copenhagen, Denmark: Lundbeck; 2012. [Available online at: http://investor.lundbeck.com/releasedetail.cfm?ReleaseID=702629; cited 27 August 2014] [Google Scholar]
- 22.United States Department of Health and Human Services, Food and Drug Administration (fda) Bendamustine hydrochloride [Web page] Silver Spring, MD: FDA; 2010. [Available at: http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm129215.htm; cited 27 August 2014] [Google Scholar]
- 23.European Medicines Agency (ema) Questions and Answers on Levact and Associated Names (Bendamustine Hydrochloride, 2.5 mg/mL, Powder for Concentrate for Solution for Infusion). Outcome of a Procedure Under Article 29 of Directive 2001/83/EC as amended. London, UK: EMA; 2010. [Available online at: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Levact_29/WC500075906.pdf; cited 27 August 2014] [Google Scholar]
- 24.Kahl BS, Bartlett NL, Leonard JP, et al. Bendamustine is effective therapy in patients with rituximab-refractory, indolent B-cell non-Hodgkin lymphoma: results from a multicenter study. Cancer. 2010;116:106–14. doi: 10.1002/cncr.24714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus chop plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–10. doi: 10.1016/S0140-6736(12)61763-2. [DOI] [PubMed] [Google Scholar]
- 26.Knauf WU, Lissichkov T, Aldaoud A, et al. Phase iii randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27:4378–84. doi: 10.1200/JCO.2008.20.8389. [DOI] [PubMed] [Google Scholar]
- 27.Swerdlow S, Campo E, Harris N, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 28.Oken M, Creech R, Tormey D, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Binet JL, Auquier A, Dighiero G, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48:198–206. doi: 10.1002/1097-0142(19810701)48:1<198::AID-CNCR2820480131>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 30.Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–34. doi: 10.1182/blood-2016-08-737650. [DOI] [PubMed] [Google Scholar]
- 31.Cephalon Inc. Highlights of Prescribing Information: Treanda (Bendamustine Hydrochloride) Injection, for Intravenous Infusion. Frazer, PA: Cephalon Inc.; 2008. [Google Scholar]
- 32.Lundbeck Canada Inc. Treanda (Bendamustine Hydrochloride for Injection) [product monograph] Montreal, QC: Lundbeck Canada; 2013. [Google Scholar]
- 33.United States, Department of Health and Human Services, National Institutes of Health, National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE) Washington, DC: Department of Health and Human Services; 2010. Ver. 4.0. [Available online at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf; cited 27 August 2014] [Google Scholar]
- 34.Friedberg JW, Cohen P, Chen L, et al. Bendamustine in patients with rituximab-refractory indolent and transformed non-Hodgkin’s lymphoma: results from a phase ii multi-center, single-agent study. J Clin Oncol. 2008;26:204–10. doi: 10.1200/JCO.2007.12.5070. [DOI] [PubMed] [Google Scholar]
- 35.Heider A, Niederle N. Efficacy and toxicity of bendamustine in patients with relapsed low-grade non-Hodgkin’s lymphomas. Anticancer Drugs. 2001;12:725–9. doi: 10.1097/00001813-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Cheson BD, Wendtner CM, Pieper A, et al. Optimal use of bendamustine in chronic lymphocytic leukemia, non-Hodgkin lymphomas, and multiple myeloma: treatment recommendations from an international consensus panel. Clin Lymphoma Myeloma Leuk. 2010;10:21–7. doi: 10.3816/CLML.2010.n.002. [DOI] [PubMed] [Google Scholar]
- 37.Koolwine J, Crosbie T, Gazze G, Turner R, Wiernikowski J, Assaily W. A Canadian perspective on the safe administration of bendamustine and the prevention and management of adverse events. Curr Oncol. 2014;21:35–42. doi: 10.3747/co.21.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sorensen E. Managing patients with indolent lymphoma treated with bendamustine: a nursing perspective. Clin J Oncol Nurs. 2013;17:303–11. doi: 10.1188/13.CJON.303-311. [DOI] [PubMed] [Google Scholar]
- 39.Brugger W, Ghielmini M. Bendamustine in indolent non-Hodgkin’s lymphoma: a practice guide for patient management. Oncologist. 2013;18:954–64. doi: 10.1634/theoncologist.2013-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kouroukis C, Chia S, Verma S, et al. Canadian supportive care recommendations for the management of neutropenia in patients with cancer. Curr Oncol. 2008;15:9–23. doi: 10.3747/co.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rummel MJ, Balser C, Kaiser U, et al. Bendamustine plus rituximab versus fludarabine plus rituximab in patients with relapsed follicular, indolent, or mantle cell lymphomas—8-year follow-up results of the randomized phase iii study nhl 2-2003 on behalf of the stil (Study Group Indolent Lymphomas, Germany) [abstract 145]. Abstracts and Program [Web site] of the 56th American Society of Hematology Annual Meeting and Exposition; San Francisco, CA, U.S.A.. 6–9 December 2014; [Available at: https://ash.confex.com/ash/2014/webprogram/Paper69154.html; cited 30 May 2015] [Google Scholar]
- 42.Alberta Health Services . Lymphoma. Edmonton, AB: Alberta Health Services; 2014. Clinical Practice Guideline LYHE-002. Ver. 8. [Google Scholar]
- 43.BC Cancer Agency (bcca) Health Professionals > Professional Resources > Cancer Management Guidelines > Lymphoma, Chronic Leukemia, Myeloma Cancer > Lymphoma [Web resource] Vancouver, BC: BCCA; 2013. [Available at: http://www.bccancer.bc.ca/health-professionals/professional-resources/cancer-management-guidelines/lymphoma-chronic-leukemia-myeloma/lymphoma; cited 8 June 2015] [Google Scholar]
- 44.Kuruvilla J, Assouline S, Hodgson D, et al. A Canadian evidence-based guideline for the first-line treatment of follicular lymphoma: joint consensus of the Lymphoma Canada Scientific Advisory Board. Clin Lymphoma Myeloma Leuk. 2015;15:59–74. doi: 10.1016/j.clml.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 45.Rummel MJ, Maschmeyer G, Ganser A, et al. Bendamustine plus rituximab (b-r) versus chop plus rituximab (chop-r) as first-line treatment in patients with indolent and mantle cell lymphomas (mcl)—7 year updated results from the stil nhl1 study [abstract 4407]. Abstracts and Program [Web site] of the 56th American Society of Hematology Annual Meeting and Exposition; San Francisco, CA, U.S.A.. 6–9 December 2014; [Available at: https://ash.confex.com/ash/2014/webprogram/Paper69997.html; cited 30 May 2015] [Google Scholar]
- 46.Eichhorst B, Fink AM, Busch R, et al. Frontline chemoimmunotherapy with fludarabine (f), cyclophosphamide (c), and rituximab (r) (fcr) shows superior efficacy in comparison to bendamustine (b) and rituximab (br) in previously untreated and physically fit patients (pts) with advanced chronic lymphocytic leukemia (cll): final analysis of an international, randomized study of the German CLL Study Group (gcllsg) (cll10 study) [abstract 19]. Abstracts and Program [Web site] of the 56th American Society of Hematology Annual Meeting and Exposition; San Francisco, CA, U.S.A.. 6–9 December 2014; [Available at: https://ash.confex.com/ash/2014/webprogram/Paper69485.html; cited 30 May 2015] [Google Scholar]
- 47.Alberta Health Services . Chronic Lymphocytic Leukemia. Edmonton, AB: Alberta Health Services; 2014. Clinical practice guideline LYHE-007. Ver. 3. [Available online at: http://www.albertahealthservices.ca/hp/if-hp-cancer-guide-lyhe007.pdf; cited 24 November 2014] [Google Scholar]