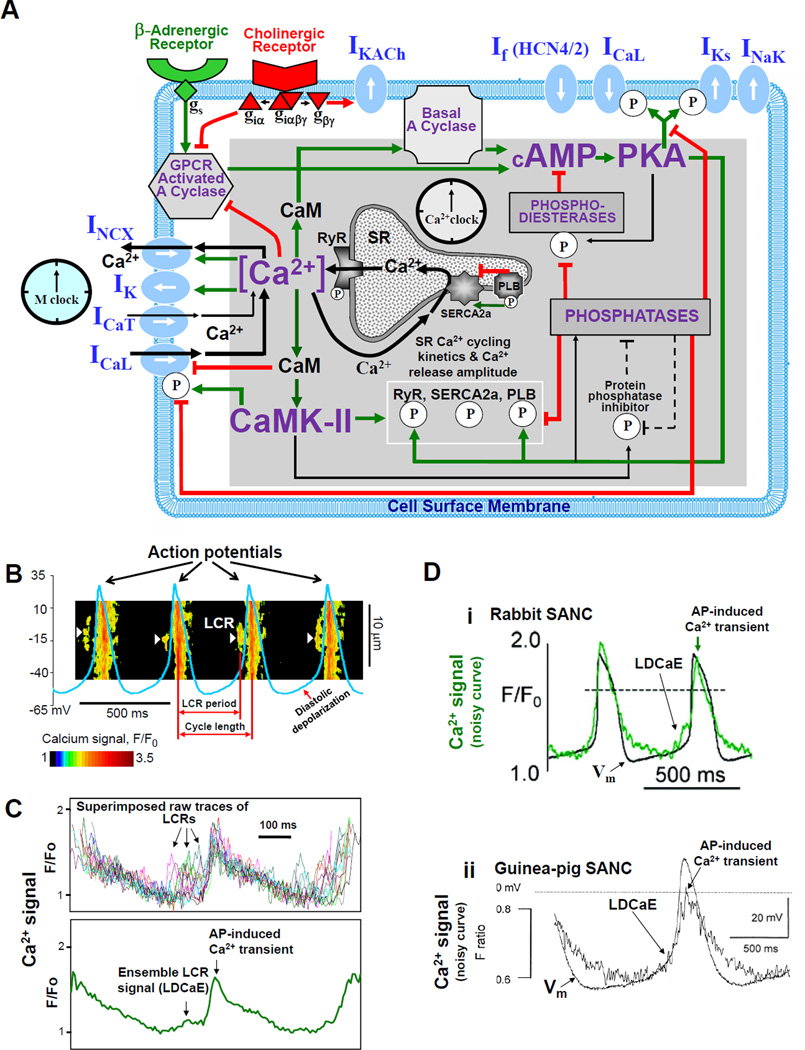
Figure 1.
SA node cell is a coupled-clock system of voltage membrane clock (M-clock) and an intracellular, sarcoplasmic reticulum (SR)-based Ca2+ clock. A: Schematic illustration of interactions of key molecules comprising the system (PLB = phospholamban). Note that common regulatory factors (purple large lettering) govern the function of both the Ca2+-clock (gray intracellular area) and the M-clock (light-blue cell membrane area with blue labels depicting electrogenic proteins). These common factors act as nodes within the system to couple the function of both clocks activities. The system is balanced as illustrated by traffic-light-like colors: green arrows designate signaling driving action potential (AP) firing but red lines show suppression, maintaining a given steady-state level of cAMP and protein phosphorylation. G protein-coupled receptors (green and red shapes within the membrane) modulate both the Ca2+-clock and M-clock function via those same crucial signaling nodes of the system. Modified from (11). B: Definition of diastolic depolarization, local Ca2+ releases (LCRs), LCR period, and cycle length. Line-scan image of LCRs (white arrows) is superimposed with spontaneous APs recorded in rabbit SANC. C: upper panel, LCRs imaged by confocal microscopy; lower panel, temporal average of the LCRs creates ensemble LCR signal or Late Diastolic Ca2+ Elevation (LDCaE) that precedes AP-induced Ca2+ transient. D: LDCaE in single SANC of rabbit (sub-panel I, modified from (53)) and guinea-pig (sub-panel ii, modified from (51), published with permission).