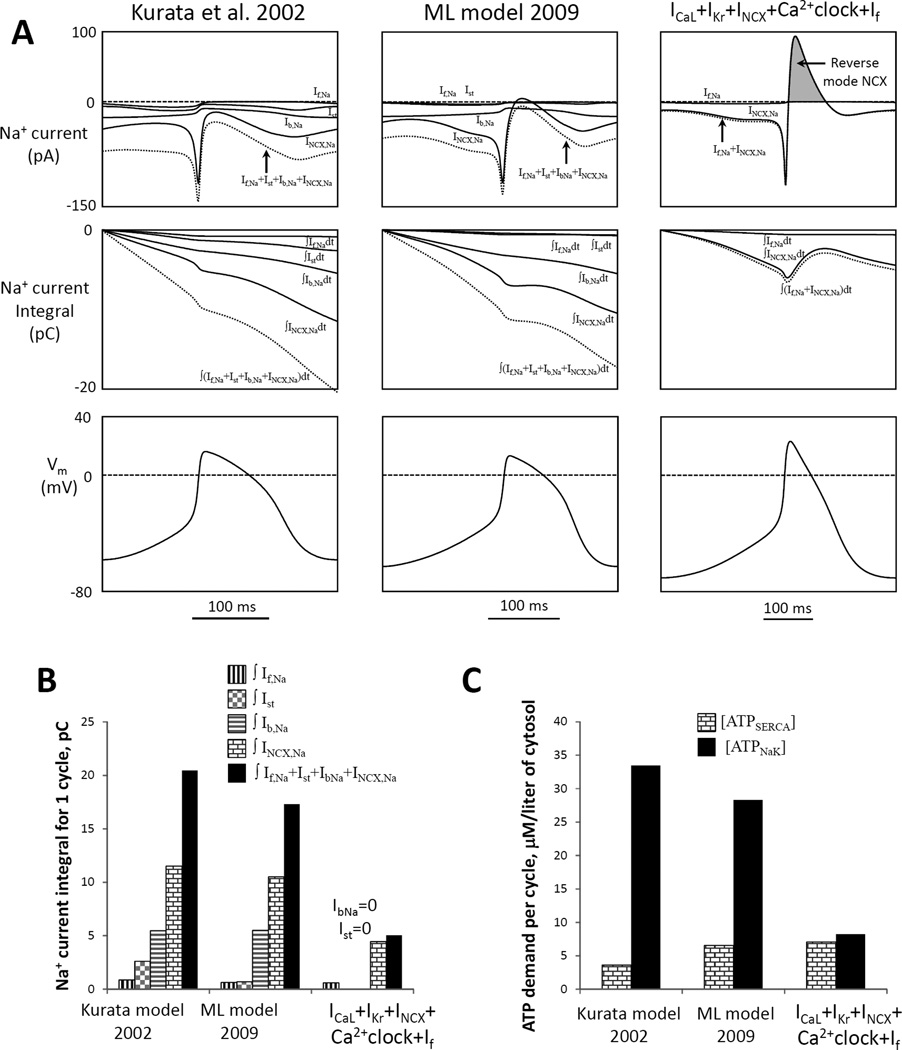
Figure 8.
A flexible and robust pacemaker model with minimal set of components (44) features a small steady-state influx of Na+ and is expected to be more energy-efficient in terms of Na+ balance regulation (i.e. requires less ATP-dependent Na+ extrusion) vs. prior models Kurata et al. (66) and Maltsev and Lakatta (ML) (10). A: Top panels show simulations of trans-membrane Na+ currents via different mechanisms (If,Na, Ist, Ib,Na, INCX,Na) and their sum (dotted lines) in different models for one AP cycle (MDP-to-MDP, bottom panels). Middle panels: respective time-dependent integrals of Na+ currents of the top panels reflecting Na+ accumulation (during one AP cycle) in the absence of Na+ extrusion by ATPases. B: Respective integrals of Na+ currents in panel A calculated for one AP cycle. All three models have cell electric capacitance of 32 pF. C: Cell energy budget to maintain Na+ homeostasis [ATPNaK] and to pump Ca2+ to the SR [ATPSERCA] estimated by three models of SANC. From (44).