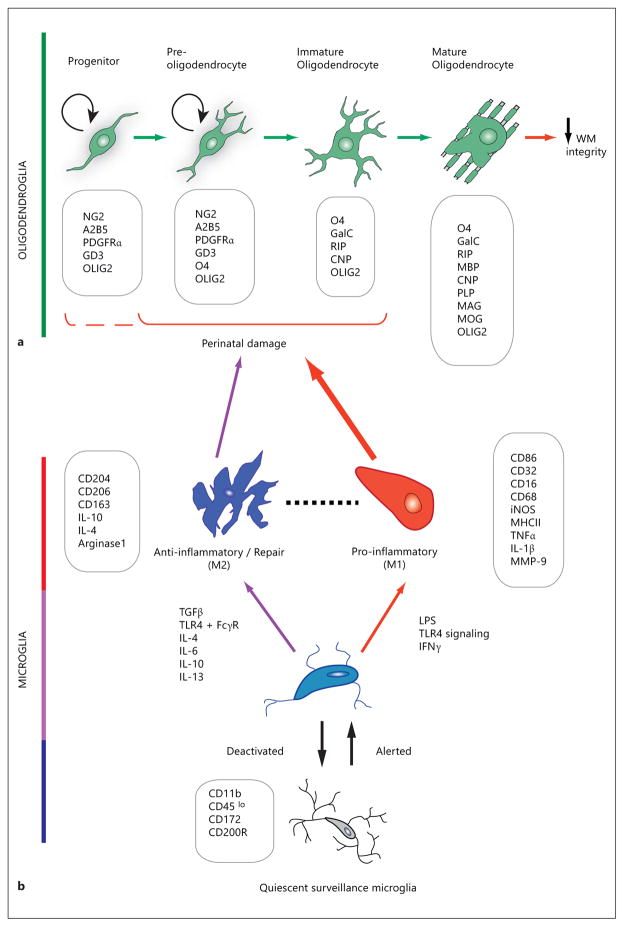
Fig. 1.
Schematic representation of the changes in morphology and gene expression during oligodendroglial development (a) and microglial activation (b), with characteristic stage- or state-specific proteins presented in boxes. Exposure of susceptible cells (a) to activated cells (b) contributes to changes in WM integrity. a Damage to immature stages of the oligodendroglial lineage leads to compromised WM integrity. The OPC develops from a neural precursor as a proliferative, migratory cell which expresses surface antigens (e.g. NG2, PDGFR-α) that are used as identifying markers to distinguish them from the postmitotic, morphologically complex and increasingly membranous immature oligodendrocyte and mature oligodendrocyte. Arrows between developmental stages in oligodendroglia denote the direction of lineage progression during maturation. OPCs and preoligodendrocytes possess the capacity for self-renewal (circular arrows), whereas this ability is lost in immature and mature oligodendrocytes. The oligodendroglial lineage is characterized by monoclonal antibodies against surface gangliosides (GD3, A2B5, RIP), sulfatides (O4), and the galactolipid galactocerebroside (GalC). Progenitors are further identified by the NG2 proteoglycan and platelet-derived growth factor receptor-alpha (PDGFRα). Myelin-specific proteins are abbreviated as follows: CNP = 2′,3′-cyclic nucleotide 3′-phosphodiesterase; MAG = myelin-associated glycoprotein; MOG = myelin oligodendrocyte glycoprotein. The nuclear transcription factor OLIG2 is widely used for the histological identification of oligodendroglial cells, because of its lineage-restricted expression throughout oligodendrocyte progenitor development. The O4+ stage oligodendroglial cell has been found to be particularly susceptible to perinatal damage, as shown with the solid bracket [201]. The possibility of hyperoxia-induced damage to NG2+ cells [176] has been included by the dashed partial bracket. b Resting or ramified microglia receive environmental signals that transform the surveying microglia to an activated phenotype. Microglia under activation and deactivation, and distinct polarizing stimuli may induce either classically activated, proinflammatory M1 state or alternatively activated anti-inflammatory M2 state. M1 macrophage/microglia produces proinflammatory cytokines and oxidative metabolites, and may damage healthy neural cells while protecting against tumors and pathogens. M2 microglia are anti-inflammatory, and promote tissue repair, remodeling and angiogenesis. The dashed line between M1 and M2 denotes less easily defined intermediate phenotypes. Boxes list CD antigen proteins, enzymes and cytokines whose expression and/or secretion characterize distinct microglial activation states. MMP = Matrix metalloproteinase; TGF-β = transforming growth factor β; FcγR = Fcγ receptor [78, 83].