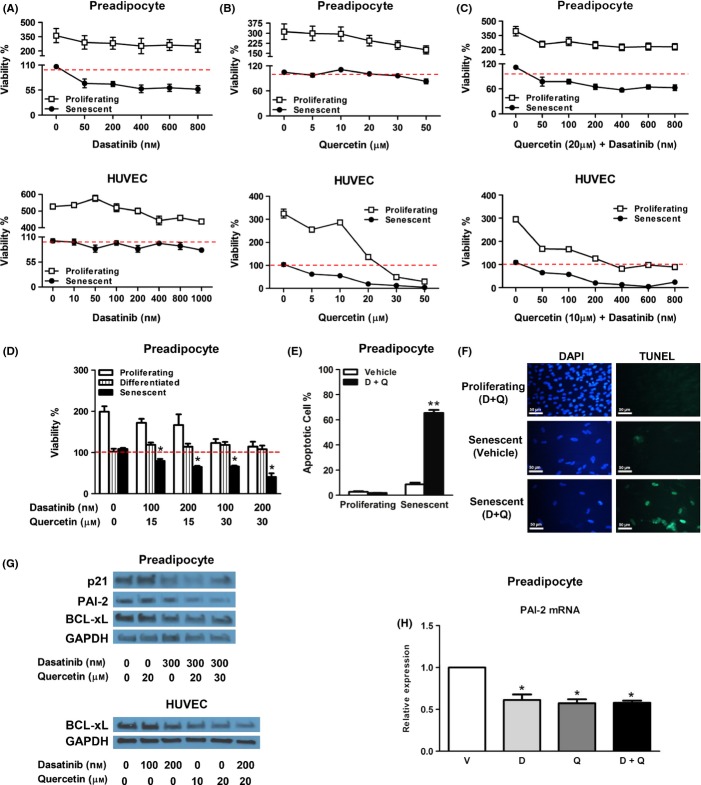
Fig 2.
Dasatinib and quercetin target senescent cells. (A) D is more effective in selectively reducing viability (ATPLite) of senescent preadipocytes than HUVECs. Preadipocytes and HUVECs were exposed to different concentrations of D for 3 days. The red line denotes plating densities on day 0 of nondividing senescent (set to 100%) as well as proliferating nonsenescent cells (also set to 100%). Preadipocyte data are means ± SEM of four experiments in each of four different subjects. HUVEC data are means ± SEM of five replicates at each concentration. (B) Q is more effective in selectively reducing viability (ATPLite) of senescent HUVECs than preadipocytes. Proliferating and senescent preadipocytes and HUVECs were exposed to different concentrations of Q for 3 days. Preadipocyte data are means ± SEM of four experiments in each of four different subjects. HUVEC data are means ± SEM of five replicates at each concentration. (C) Combining D and Q selectively reduced viability of both senescent preadipocytes and senescent HUVECs. Proliferating and senescent preadipocytes and HUVECs were exposed to a fixed concentration of Q and different concentrations of D for 3 days. Optimal Q concentrations for inducing death of senescent preadipocyte and HUVEC cells were 20 and 10 μm, respectively. (D) D and Q do not affect the viability of quiescent fat cells. Nonsenescent preadipocytes (proliferating) as well as nonproliferating, nonsenescent differentiated fat cells prepared from preadipocytes (differentiated), as well as nonproliferating preadipocytes that had been exposed to 10 Gy radiation 25 days before to induce senescence (senescent) were treated with D+Q for 48 h. N = 6 preadipocyte cultures isolated from different subjects. *P < 0.05; anova. 100% indicates ATPLite intensity at day 0 for each cell type and the bars represent the ATPLite intensity after 72 h. The drugs resulted in lower ATPLite in proliferating cells than in vehicle-treated cells after 72 h, but ATPLite intensity did not fall below that at day 0. This is consistent with inhibition of proliferation, and not necessarily cell death. Fat cell ATPLite was not substantially affected by the drugs, consistent with lack of an effect of even high doses of D+Q on nonproliferating, differentiated cells. ATPLite was lower in senescent cells exposed to the drugs for 72 h than at plating on day 0. As senescent cells do not proliferate, this indicates that the drugs decrease senescent cell viability. (E, F) D and Q cause more apoptosis of senescent than nonsenescent primary human preadipocytes (terminal deoxynucleotidyl transferase dUTP nick end labeling [TUNEL] assay). (E) D (200 nM) plus Q (20 μm) resulted in 65% apoptotic cells (TUNEL assay) after 12 h in senescent but not proliferating, nonsenescent preadipocyte cultures. Cells were from three subjects; four replicates; **P < 0.0001; anova. (F) Primary human preadipocytes were stained with DAPI to show nuclei or analyzed by TUNEL to show apoptotic cells. Senescence was induced by 10 Gy radiation 25 days previously. Proliferating, nonsenescent cells were exposed to D+Q for 24 h, and senescent cells from the same subjects were exposed to vehicle or D+Q. D+Q induced apoptosis in senescent, but not nonsenescent, cells (compare the green in the upper to lower right panels). The bars indicate 50 μm. (G) Effect of vehicle, D, Q, or D+Q on nonsenescent preadipocyte and HUVEC p21, BCL-xL, and PAI-2 by Western immunoanalysis. (H) Effect of vehicle, D, Q, or D+Q on preadipocyte on PAI-2 mRNA by PCR. N = 3; *P < 0.05; anova.