Abstract
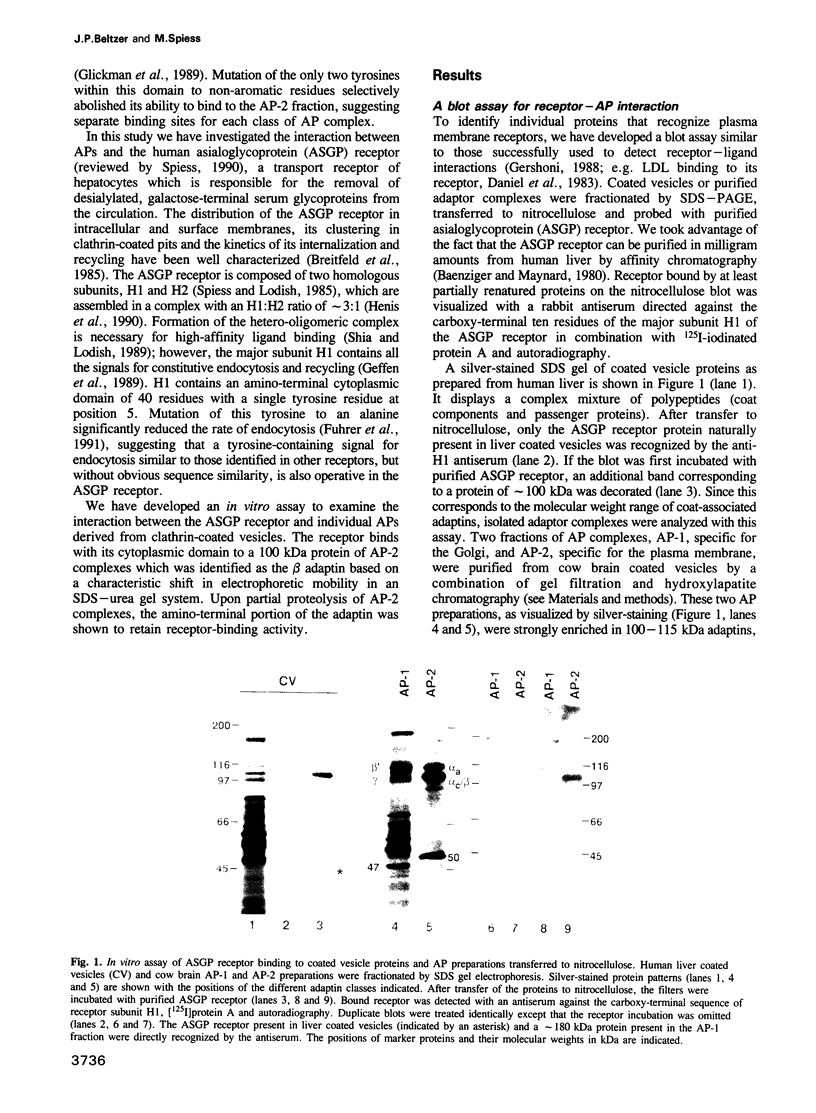
The asialoglycoprotein (ASGP) receptor was used to probe total clathrin-coated vesicle proteins and purified adaptor proteins (APs) which had been fractionated by gel electrophoresis and transferred to nitrocellulose. The receptor was found to interact with proteins of approximately 100 kDa. The cytoplasmic domain of the ASGP receptor subunit H1 fused to dihydrofolate reductase competed for receptor binding to the 100 kDa polypeptide in the plasma membrane-type AP complexes (AP-2). A fusion protein containing the cytoplasmic domain of the endocytic mutant haemagglutinin HA-Y543 also competed, but a protein with the wild-type haemagglutinin sequence did not. This indicates that the observed interaction is specific for the cytoplasmic domain of the receptor and involves the tyrosine signal for endocytosis. When fractionated by gel electrophoresis in the presence of urea, the ASGP receptor binding polypeptide displayed a characteristic shift in electrophoretic mobility identifying it as the beta adaptin. Partial proteolysis of the AP-2 preparation followed by the receptor binding assay revealed that the aminoterminal domain of the beta adaptin contains the binding site for receptors.
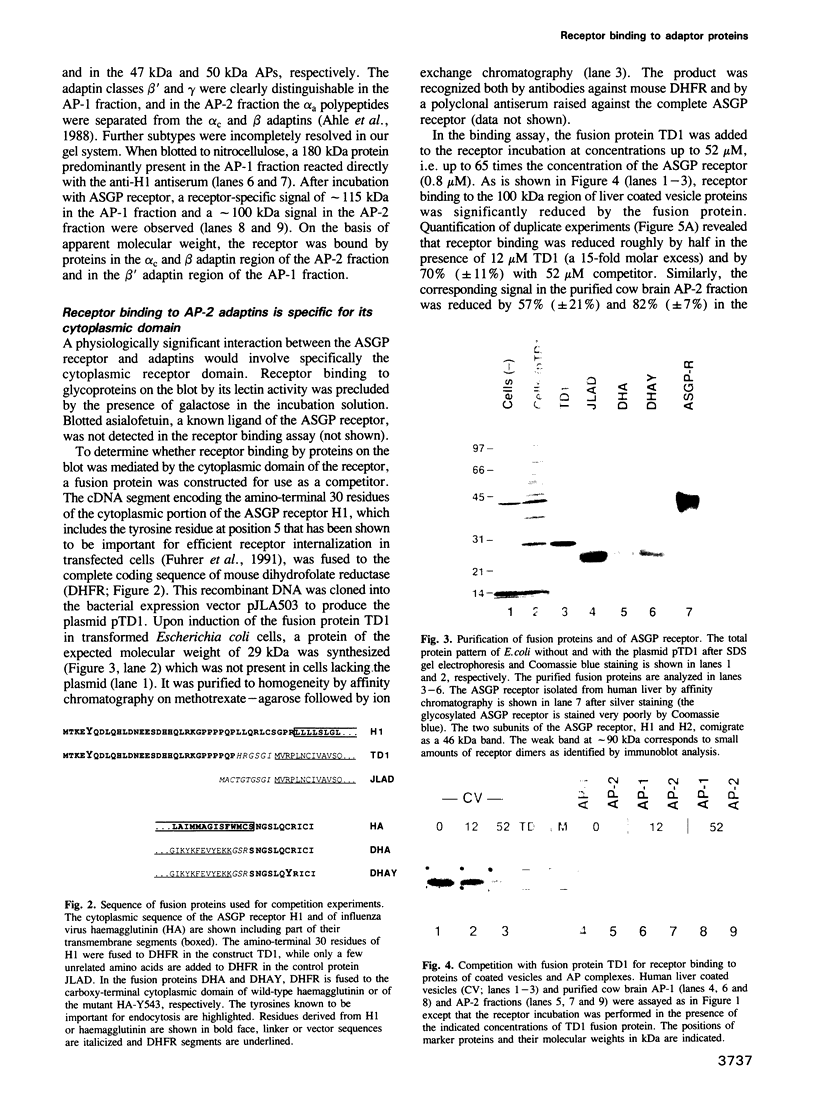
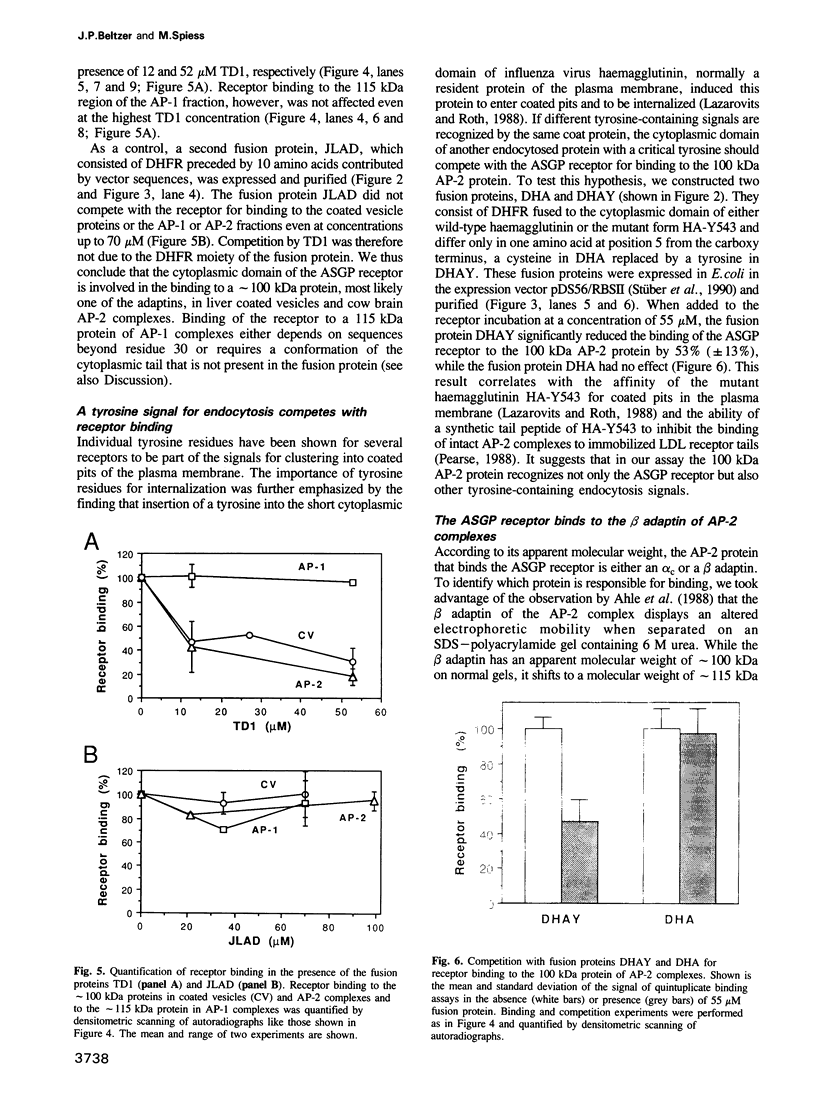
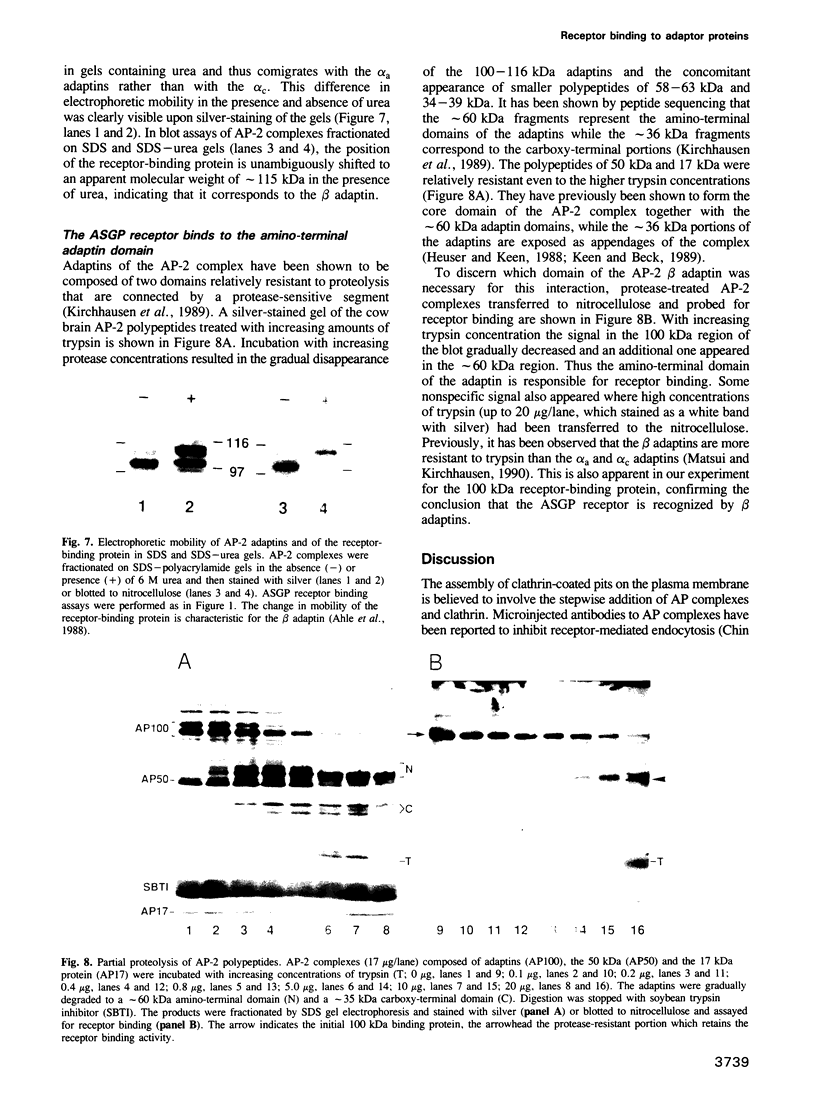
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahle S., Mann A., Eichelsbacher U., Ungewickell E. Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. EMBO J. 1988 Apr;7(4):919–929. doi: 10.1002/j.1460-2075.1988.tb02897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahle S., Ungewickell E. Identification of a clathrin binding subunit in the HA2 adaptor protein complex. J Biol Chem. 1989 Nov 25;264(33):20089–20093. [PubMed] [Google Scholar]
- Alvarez E., Gironès N., Davis R. J. A point mutation in the cytoplasmic domain of the transferrin receptor inhibits endocytosis. Biochem J. 1990 Apr 1;267(1):31–35. doi: 10.1042/bj2670031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge W. Fast and sensitive detection of protein and DNA bands by treatment with potassium permanganate. J Biochem Biophys Methods. 1985 May;11(1):13–20. doi: 10.1016/0165-022x(85)90037-5. [DOI] [PubMed] [Google Scholar]
- Baenziger J. U., Maynard Y. Human hepatic lectin. Physiochemical properties and specificity. J Biol Chem. 1980 May 25;255(10):4607–4613. [PubMed] [Google Scholar]
- Breitfeld P. P., Simmons C. F., Jr, Strous G. J., Geuze H. J., Schwartz A. L. Cell biology of the asialoglycoprotein receptor system: a model of receptor-mediated endocytosis. Int Rev Cytol. 1985;97:47–95. doi: 10.1016/s0074-7696(08)62348-7. [DOI] [PubMed] [Google Scholar]
- Chen W. J., Goldstein J. L., Brown M. S. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990 Feb 25;265(6):3116–3123. [PubMed] [Google Scholar]
- Chin D. J., Straubinger R. M., Acton S., Näthke I., Brodsky F. M. 100-kDa polypeptides in peripheral clathrin-coated vesicles are required for receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9289–9293. doi: 10.1073/pnas.86.23.9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collawn J. F., Stangel M., Kuhn L. A., Esekogwu V., Jing S. Q., Trowbridge I. S., Tainer J. A. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell. 1990 Nov 30;63(5):1061–1072. doi: 10.1016/0092-8674(90)90509-d. [DOI] [PubMed] [Google Scholar]
- Daniel T. O., Schneider W. J., Goldstein J. L., Brown M. S. Visualization of lipoprotein receptors by ligand blotting. J Biol Chem. 1983 Apr 10;258(7):4606–4611. [PubMed] [Google Scholar]
- Davis C. G., Lehrman M. A., Russell D. W., Anderson R. G., Brown M. S., Goldstein J. L. The J.D. mutation in familial hypercholesterolemia: amino acid substitution in cytoplasmic domain impedes internalization of LDL receptors. Cell. 1986 Apr 11;45(1):15–24. doi: 10.1016/0092-8674(86)90533-7. [DOI] [PubMed] [Google Scholar]
- Davis C. G., van Driel I. R., Russell D. W., Brown M. S., Goldstein J. L. The low density lipoprotein receptor. Identification of amino acids in cytoplasmic domain required for rapid endocytosis. J Biol Chem. 1987 Mar 25;262(9):4075–4082. [PubMed] [Google Scholar]
- Endo T., Schatz G. Latent membrane perturbation activity of a mitochondrial precursor protein is exposed by unfolding. EMBO J. 1988 Apr;7(4):1153–1158. doi: 10.1002/j.1460-2075.1988.tb02925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffen I., Wessels H. P., Roth J., Shia M. A., Spiess M. Endocytosis and recycling of subunit H1 of the asialoglycoprotein receptor is independent of oligomerization with H2. EMBO J. 1989 Oct;8(10):2855–2861. doi: 10.1002/j.1460-2075.1989.tb08433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoni J. M. Protein blotting: a manual. Methods Biochem Anal. 1988;33:1–58. doi: 10.1002/9780470110546.ch1. [DOI] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Luzio J. P., Schwartz A. L. A cycloheximide-resistant pool of receptors for asialoglycoproteins and mannose 6-phosphate residues in the Golgi complex of hepatocytes. EMBO J. 1984 Nov;3(11):2677–2685. doi: 10.1002/j.1460-2075.1984.tb02193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman J. N., Conibear E., Pearse B. M. Specificity of binding of clathrin adaptors to signals on the mannose-6-phosphate/insulin-like growth factor II receptor. EMBO J. 1989 Apr;8(4):1041–1047. doi: 10.1002/j.1460-2075.1989.tb03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg D. F., Wager R. E., Farrell D. C., Hildreth J., 4th, Quesenberry M. S., Loeb J. A., Holland E. C., Drickamer K. Major and minor forms of the rat liver asialoglycoprotein receptor are independent galactose-binding proteins. Primary structure and glycosylation heterogeneity of minor receptor forms. J Biol Chem. 1987 Jul 15;262(20):9828–9838. [PubMed] [Google Scholar]
- Henis Y. I., Katzir Z., Shia M. A., Lodish H. F. Oligomeric structure of the human asialoglycoprotein receptor: nature and stoichiometry of mutual complexes containing H1 and H2 polypeptides assessed by fluorescence photobleaching recovery. J Cell Biol. 1990 Oct;111(4):1409–1418. doi: 10.1083/jcb.111.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Keen J. Deep-etch visualization of proteins involved in clathrin assembly. J Cell Biol. 1988 Sep;107(3):877–886. doi: 10.1083/jcb.107.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Schatz G. The cleavable prepiece of an imported mitochondrial protein is sufficient to direct cytosolic dihydrofolate reductase into the mitochondrial matrix. FEBS Lett. 1984 Dec 10;178(2):306–310. doi: 10.1016/0014-5793(84)80622-5. [DOI] [PubMed] [Google Scholar]
- Iacopetta B. J., Rothenberger S., Kühn L. C. A role for the cytoplasmic domain in transferrin receptor sorting and coated pit formation during endocytosis. Cell. 1988 Aug 12;54(4):485–489. doi: 10.1016/0092-8674(88)90069-4. [DOI] [PubMed] [Google Scholar]
- Jing S. Q., Spencer T., Miller K., Hopkins C., Trowbridge I. S. Role of the human transferrin receptor cytoplasmic domain in endocytosis: localization of a specific signal sequence for internalization. J Cell Biol. 1990 Feb;110(2):283–294. doi: 10.1083/jcb.110.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen J. H., Beck K. A. Identification of the clathrin-binding domain of assembly protein AP-2. Biochem Biophys Res Commun. 1989 Jan 16;158(1):17–23. doi: 10.1016/s0006-291x(89)80170-6. [DOI] [PubMed] [Google Scholar]
- Keen J. H. Clathrin assembly proteins: affinity purification and a model for coat assembly. J Cell Biol. 1987 Nov;105(5):1989–1998. doi: 10.1083/jcb.105.5.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen J. H., Willingham M. C., Pastan I. H. Clathrin-coated vesicles: isolation, dissociation and factor-dependent reassociation of clathrin baskets. Cell. 1979 Feb;16(2):303–312. doi: 10.1016/0092-8674(79)90007-2. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T., Nathanson K. L., Matsui W., Vaisberg A., Chow E. P., Burne C., Keen J. H., Davis A. E. Structural and functional division into two domains of the large (100- to 115-kDa) chains of the clathrin-associated protein complex AP-2. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2612–2616. doi: 10.1073/pnas.86.8.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ktistakis N. T., Thomas D., Roth M. G. Characteristics of the tyrosine recognition signal for internalization of transmembrane surface glycoproteins. J Cell Biol. 1990 Oct;111(4):1393–1407. doi: 10.1083/jcb.111.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda S., Tanizawa K., Sakamoto Y., Tanaka H., Soda K. Alanine dehydrogenases from two Bacillus species with distinct thermostabilities: molecular cloning, DNA and protein sequence determination, and structural comparison with other NAD(P)(+)-dependent dehydrogenases. Biochemistry. 1990 Jan 30;29(4):1009–1015. doi: 10.1021/bi00456a025. [DOI] [PubMed] [Google Scholar]
- Lazarovits J., Roth M. A single amino acid change in the cytoplasmic domain allows the influenza virus hemagglutinin to be endocytosed through coated pits. Cell. 1988 Jun 3;53(5):743–752. doi: 10.1016/0092-8674(88)90092-x. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Goldstein J. L., Brown M. S., Russell D. W., Schneider W. J. Internalization-defective LDL receptors produced by genes with nonsense and frameshift mutations that truncate the cytoplasmic domain. Cell. 1985 Jul;41(3):735–743. doi: 10.1016/s0092-8674(85)80054-4. [DOI] [PubMed] [Google Scholar]
- Lobel P., Fujimoto K., Ye R. D., Griffiths G., Kornfeld S. Mutations in the cytoplasmic domain of the 275 kd mannose 6-phosphate receptor differentially alter lysosomal enzyme sorting and endocytosis. Cell. 1989 Jun 2;57(5):787–796. doi: 10.1016/0092-8674(89)90793-9. [DOI] [PubMed] [Google Scholar]
- Mahaffey D. T., Peeler J. S., Brodsky F. M., Anderson R. G. Clathrin-coated pits contain an integral membrane protein that binds the AP-2 subunit with high affinity. J Biol Chem. 1990 Sep 25;265(27):16514–16520. [PubMed] [Google Scholar]
- Manfredi J. J., Bazari W. L. Purification and characterization of two distinct complexes of assembly polypeptides from calf brain coated vesicles that differ in their polypeptide composition and kinase activities. J Biol Chem. 1987 Sep 5;262(25):12182–12188. [PubMed] [Google Scholar]
- Matsui W., Kirchhausen T. Stabilization of clathrin coats by the core of the clathrin-associated protein complex AP-2. Biochemistry. 1990 Dec 4;29(48):10791–10798. doi: 10.1021/bi00500a011. [DOI] [PubMed] [Google Scholar]
- Moore M. S., Mahaffey D. T., Brodsky F. M., Anderson R. G. Assembly of clathrin-coated pits onto purified plasma membranes. Science. 1987 May 1;236(4801):558–563. doi: 10.1126/science.2883727. [DOI] [PubMed] [Google Scholar]
- Mostov K. E., de Bruyn Kops A., Deitcher D. L. Deletion of the cytoplasmic domain of the polymeric immunoglobulin receptor prevents basolateral localization and endocytosis. Cell. 1986 Nov 7;47(3):359–364. doi: 10.1016/0092-8674(86)90592-1. [DOI] [PubMed] [Google Scholar]
- Pearse B. M. Assembly of the mannose-6-phosphate receptor into reconstituted clathrin coats. EMBO J. 1985 Oct;4(10):2457–2460. doi: 10.1002/j.1460-2075.1985.tb03956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse B. M. Receptors compete for adaptors found in plasma membrane coated pits. EMBO J. 1988 Nov;7(11):3331–3336. doi: 10.1002/j.1460-2075.1988.tb03204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K., Keen J. H. Interaction of assembly protein AP-2 and its isolated subunits with clathrin. Biochemistry. 1991 Jun 4;30(22):5590–5597. doi: 10.1021/bi00236a036. [DOI] [PubMed] [Google Scholar]
- Prywes R., Livneh E., Ullrich A., Schlessinger J. Mutations in the cytoplasmic domain of EGF receptor affect EGF binding and receptor internalization. EMBO J. 1986 Sep;5(9):2179–2190. doi: 10.1002/j.1460-2075.1986.tb04482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. S. 100-kD coated vesicle proteins: molecular heterogeneity and intracellular distribution studied with monoclonal antibodies. J Cell Biol. 1987 Apr;104(4):887–895. doi: 10.1083/jcb.104.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberger S., Iacopetta B. J., Kühn L. C. Endocytosis of the transferrin receptor requires the cytoplasmic domain but not its phosphorylation site. Cell. 1987 May 8;49(3):423–431. doi: 10.1016/0092-8674(87)90295-9. [DOI] [PubMed] [Google Scholar]
- Schauder B., Blöcker H., Frank R., McCarthy J. E. Inducible expression vectors incorporating the Escherichia coli atpE translational initiation region. Gene. 1987;52(2-3):279–283. doi: 10.1016/0378-1119(87)90054-0. [DOI] [PubMed] [Google Scholar]
- Schröder S., Ungewickell E. Subunit interaction and function of clathrin-coated vesicle adaptors from the Golgi and the plasma membrane. J Biol Chem. 1991 Apr 25;266(12):7910–7918. [PubMed] [Google Scholar]
- Shia M. A., Lodish H. F. The two subunits of the human asialoglycoprotein receptor have different fates when expressed alone in fibroblasts. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1158–1162. doi: 10.1073/pnas.86.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess M., Lodish H. F. An internal signal sequence: the asialoglycoprotein receptor membrane anchor. Cell. 1986 Jan 17;44(1):177–185. doi: 10.1016/0092-8674(86)90496-4. [DOI] [PubMed] [Google Scholar]
- Spiess M., Lodish H. F. Sequence of a second human asialoglycoprotein receptor: conservation of two receptor genes during evolution. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6465–6469. doi: 10.1073/pnas.82.19.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijderhand-Bleekemolen J. E., Schwartz A. L., Slot J. W., Strous G. J., Geuze H. J. Ligand- and weak base-induced redistribution of asialoglycoprotein receptors in hepatoma cells. J Cell Biol. 1987 Jun;104(6):1647–1654. doi: 10.1083/jcb.104.6.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]