Abstract
Telomere length shortens with aging, and short telomeres have been linked to a wide variety of pathologies. Previous studies suggested a discrepancy in age-associated telomere shortening rate estimated by cross-sectional studies versus the rate measured in longitudinal studies, indicating a potential bias in cross-sectional estimates. Intergenerational changes in initial telomere length, such as that predicted by the previously described effect of a father’s age at birth of his offspring (FAB), could explain the discrepancy in shortening rate measurements. We evaluated whether changes occur in initial telomere length over multiple generations in three large datasets and identified paternal birth year (PBY) as a variable that reconciles the difference between longitudinal and cross-sectional measurements. We also clarify the association between FAB and offspring telomere length, demonstrating that this effect is substantially larger than reported in the past. These results indicate the presence of a downward secular trend in telomere length at birth over generational time with potential public health implications.
Keywords: aging, genetics, human, parental effects, secular trend, telomeres, telomerase, telomere length
Introduction
The termini of linear chromosomes are shielded from recognition as DNA double-strand breaks by telomeres, TTAGGG repeats that recruit a group of protective protein accessory factors, the shelterin complex (de Lange, 2010). In humans, most normal somatic cells do not express telomerase, the enzyme capable of maintaining telomeres, a ribonucleoprotein reverse transcriptase. Therefore, normal telomerase-negative cells demonstrate progressive telomere shortening over time in all human somatic tissues (Wright et al., 1996). This eventually triggers cell growth arrest, termed ‘replicative senescence,’ via an uncapped telomere end recognized by the p53 pathway (Chin et al., 1999). Telomere shortening occurs both in vitro and in vivo, even in cell populations derived from telomerase-positive stem cell compartments such as leukocytes (Daniali et al., 2013). Leukocyte telomere length (LTL) shortens with age in humans, and reduced LTL has been linked to a host of pathologies such as dementia, hypertension, cardiovascular events, asthma, diabetes, and stroke as well as environmental factors, such as smoking, obesity, chronic infections, and stress (reviewed in Bojesen (2013)).
Because telomere length correlates with both these pathologies and environmental exposures known to drive them, telomere length may be an important indicator of general health, as an integrator of total physiological stress. Indeed, LTL may be a better indicator of biological age than chronological age and telomere measurements in leukocytes reflect systemic telomere length in other tissues (Daniali et al., 2013). Estimates of average telomere lengths for individuals of a given age as well as telomere shortening rates are predominantly based on cross-sectional analysis, large-scale measurements of individuals of differing ages. This estimation relies on the assumption that the average initial telomere length for all individuals measured does not change over time (e.g., from generation to generation). In cross-sectional studies, each individual is only measured once, so all differences in telomere length with age are ascribed to aging itself, even if generational changes have occurred. If a time-dependent change in initial telomere length, a secular trend, exists, the measurement of telomere shortening rate will be biased in the direction opposite the secular trend, leading to incorrect cross-sectional measurements of the rate of telomere shortening with age (Fig.1/Video S1). If initial/birth telomere length were increasing, cross-sectional analyses would indicate that telomere length shortens faster over time than it does in reality because younger individuals started with longer telomeres compared to older individuals, thus exaggerating age-associated differences. Conversely, if initial/birth telomere length were decreasing, cross-sectional analysis would generate aberrantly low estimates of attrition rate, as the difference between younger and older individuals would be understated. This important consideration about a secular trend in initial telomere length has not been adequately addressed.
Fig 1.
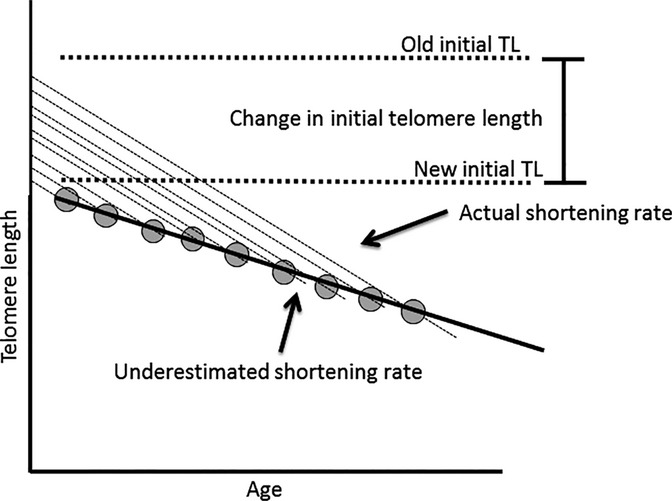
Decreasing initial telomere length understates telomere shortening rate. Cross-sectional analyses rely on the assumption that initial telomere length is not changing. In a cross-sectional estimate of telomere shortening rate (black line), the measurement of telomere length in multiple individuals of different ages (gray circles) will yield an underestimate of the actual telomere shortening rate (constant for all samples, dotted lines) if initial telomere length (y-intercept) is decreasing with time.
A small number of studies have measured telomere attrition rate longitudinally by measuring LTL in a sample population and then measuring the same individuals again at a second later time point (Gardner et al., 2005; Aviv et al., 2009; Ehrlenbach et al., 2009; Farzaneh-Far et al., 2010; Chen et al., 2011; Houben et al., 2011). Shortening is then computed as the average difference between the first and second measurement of each individual in the population. Longitudinal estimates of telomere shortening consistently produce telomere attrition rates greater than cross-sectional shortening rates by ∼20 base pairs (bp) per year. Indeed, one longitudinal study compared cross-sectional analysis of their data at each telomere measurement time point and noted this discrepancy compared with their longitudinal analysis (Chen et al., 2011). Because longitudinal studies directly observe telomere shortening in individuals, one could argue that they represent a more correct measurement of the actual telomere shortening rate. These observations lead to the conclusion that something is biasing cross-sectional studies’ measurements. If a secular trend in initial telomere length were responsible for the bias, initial telomere length would be decreasing.
Population average telomere length demonstrably can change over evolutionarily short timescales, with differing mean LTL reported between ethnic groups (Aviv et al., 2009) and countries (Eisenberg et al., 2011). Given the substantial interindividual variation in LTL of several kilobases, telomere length could easily change as a result of founder effects, as well as a passenger trait during the fixation of beneficial variants. Telomere length is inherited both genetically, as a result of polymorphisms that confer changes in telomere length such as a number of SNPs identified in the TERT gene and epigenetica-lly, as the telomere repeats themselves are directly passed to the genome of the offspring (De Meyer et al., 2014). The dual inheritance mode of telomere length is most readily observed in telomeropathies, disorders of impaired telomere maintenance (Holohan et al., 2014). Offspring of individuals with subclinical or milder forms of these diseases inherit short telomeres directly from their parents as well as the genetic polymorphism responsible for the shortening, compounding the change in telomere length and decreasing the age of onset as well as increasing the severity of the diseases (Armanios et al., 2005). This dual inheritance may generate feed-forward loops in changes in telomere length, constrained by selection at the lower end by telomeropathies and incompletely understood fitness penalties for aberrantly long telomeres, such as an increased risk of a number of cancers (Anic et al., 2013).
Many important physiological and developmental parameters are the subject of secular (time-dependent) trends in the developed world, such as decreasing age at pubertal onset (Toppari & Juul, 2010), increasing rates of obesity/diabetes and hypertension, decreased rates of smoking (Romero et al., 2012), and increasing height (Komlos & Breitfelder, 2008). All of these trends are likely to impact telomere dynamics, as they correlate with the amount and timing of cell division, sex hormones (estrogen and testosterone), nutritional state, and growth signaling, as well as exposure to cigarette smoke, all of which are known to impact telomere biology. Given the nature of these trends, an alteration in initial telomere length or population-scale telomere dynamics is quite possible.
A number of groups have reported a positive association between a father’s age at the birth of their offspring (FAB) and LTL (Unryn et al., 2005; De Meyer et al., 2007; Aston et al., 2012; Eisenberg et al., 2012). This has been linked to a positive correlation between male age and sperm telomere length in a cross-sectional analysis, showing telomere elongation in sperm cell progenitors of roughly 57 bp/year (Aston et al., 2012). The average FAB in the developed world has been increasing in the recent past (Bray et al., 2006), which should produce an increase in initial LTL. If such an increase were occurring, cross-sectional analyses would report shortening rates higher than longitudinal studies, the opposite of the trend observed. If sperm progenitor cells instead maintained their telomere length at a fixed equilibrium length and that equilibrium length had been decreasing with time in younger men, this would be observed as an age-dependent increase in a cross-sectional measurement of sperm telomere dynamics. A decreasing equilibrium sperm telomere length over time would reconcile the discrepancy between demographic trends, sperm telomere dynamics, and the difference between longitudinal and cross-sectional LTL shortening rate.
Because in most studies, subject recruitment is mostly limited to a few years, an individual’s age and birth year are typically nearly perfectly collinear. Therefore, we decided to test in three large population datasets for a change in initial telomere length by determining whether an individual’s paternal birth year (PBY), an index for time that is less collinear with age, better predicts telomere length compared to FAB when age is included in the model. We then used mediation analysis to determine to what extent the measured effects of the three variables are influenced by their collinearity in these datasets. Lastly, we performed a ‘pseudo-longitudinal’ analysis of telomere shortening in one of the datasets that included twins. We performed these analyses in the UK twin adult registry (UK Twins), the National Heart Lung and Blood Institute Family Heart Study (NHLBI-FHS), and the Asklepios study populations, which included information on FAB, PBY, age, and telomere length in 2710, 2177, and 2434 individuals, respectively. PBY predicts telomere length better than FAB in all three datasets, and models that utilize PBY instead of FAB produce age-associated shortening rates more consistent with longitudinal telomere shortening rates, indicating that a negative secular trend in initial telomere length over time could partially explain the discrepancy. Furthermore, accounting for the collinearity between those variables reveals a heterogeneous PBY effect and a larger FAB effect than previously reported.
Results
Cross-sectional estimates of telomere shortening rate are consistently lower than longitudinal estimates
Table S1 (Supporting information) summarizes the most important longitudinal studies that measured telomere shortening rate in nonarbitrary units contrasted with studies that examined attrition rate cross-sectionally in large cohorts. The weighted average telomere shortening rate in cross-sectional studies is 22.82 base pairs per year compared with 41.19 in longitudinal studies (P < 0.001).
Study characteristics for paternal birth year analyses
Telomere measurements and inclusion criteria for all three datasets have been described elsewhere (Higgins et al., 1996; Andrew et al., 2001; Rietzschel et al., 2007; Kimura et al., 2008). Paternal birth year (PBY) was collinear with age and father’s age at birth of the offspring, and age was collinear with FAB in all three datasets (Table S2), as were maternal variables; because of the collinearity between maternal and paternal effects, we could not distinguish if maternal variables contribute to the phenomena examined. Maternal effects were similar to paternal effects but of reduced statistical significance in the three populations; therefore, subsequent analyses were conducted only on paternal variables.
Both paternal birth year and father’s age at birth influence telomere length
Linear regression with terminal restriction fragment (TRF, a gel-based measure of telomere length) length as the dependent variable and either FAB or PBY in addition to age as predictors was conducted on all three datasets individually and also on all three datasets combined. For the combined dataset analysis, a categorical variable indicating dataset was included to avoid bias from interpopulation differences in mean TRF length. Because of the size of the datasets, P-values for all effects reported as significant are <0.001 unless otherwise noted.
In all three datasets, models that included PBY instead of FAB better predicted telomere length, as demonstrated by their higher overall R2 values. PBY’s fractional R2 value was also higher than FAB’s, showing that it contributed more to the model’s ability to predict telomere length (Table1). Interestingly, age’s fractional R2 value substantially decreases in models that include PBY (0.0581 in the combined PBY model compared with 0.1629 in FAB), suggesting that the effect of age in other models is partially mediated by age’s highly collinear relationship with PBY. Furthermore, age-associated shortening rates become consistent with longitudinal attrition rates when PBY is included instead of FAB (39.44 bp/year in the PBY model compared with 22.18 bp/year in the FAB model). Omission of PBY from the model could explain why previous cross-sectional analyses have arrived at erroneously low estimates of age-associated telomere shortening.
Table 1.
Comparison of paternal effect models shows that PBY can influence telomere length distinctly from FAB
| Model | Study | Paternal effect (95% CI) (bp/year) | Age effect (95% CI) (bp/year) | Paternal effect partial R2 | Age effect partial R2 | Model R2 |
|---|---|---|---|---|---|---|
| Paternal birth year (PBY) | UK Twins | −19.48 (−23.03 to −15.93) | −40.94 (−45.04 to −36.82) | 0.0681 | 0.1144 | 0.1825 |
| NHLBI-FHS | −14.66 (−18.18 to −11.14) | −36.42 (−40.54 to −32.31) | 0.0847 | 0.1114 | 0.1962 | |
| Asklepios | −17.73 (−21.97 to −13.49) | −46.05 (−52.76 to −39.33) | 0.0257 | 0.0450 | 0.0708 | |
| Combined | −17.22 (−19.40 to −15.04) | −39.44 (−42.08 to −36.80) | 0.1058 | 0.0581 | 0.3967 | |
| Father’s age at birth (FAB) | UK Twins | 13.39 (9.63 to 17.14) | −21.38 (−23.25 to −19.51) | 0.0067 | 0.1560 | 0.1627 |
| NHLBI-FHS | 14.56 (11.04 to 18.08) | −21.69 (−23.63 to −19.75) | 0.0184 | 0.1774 | 0.1958 | |
| Asklepios | 17.17 (12.90 to 21.43) | −28.30 (−33.03 to −23.57) | 0.0238 | 0.0450 | 0.0689 | |
| Combined | 14.85 (12.63 to 17.08) | −22.18 (−23.53 to −20.84) | 0.0116 | 0.1629 | 0.3911 |
Multiple linear regression models using paternal birth year (PBY) in addition to the age, top panel, produce age-associated telomere shortening rates more consistent with longitudinal measurements of telomere shortening than models that utilize father’s age at birth (FAB), bottom panel, in all three datasets individually and combined. Further, models using PBY instead of age produce higher R2 values and have a lower fractional contribution from age.
Although models including PBY instead of FAB predicted telomere length better, the model including FAB and age substantially increased the predictive power over a model including age alone in all three datasets without yielding a large decrease in fractional R2 of age to the model. This suggests that both PBY and FAB impact telomere length independent of their collinearity and that including age, PBY, and FAB in subsequent models should improve their predictive power.
Mediation analysis reveals age-associated telomere shortening rates consistent with longitudinal studies and the presence of a PBY effect distinct from FAB
Because of the observed multicollinearity of the three variables in question in all three datasets, we performed mediation analysis in order to understand how to correct for the indirect action of other variables (see Materials and methods and Fig.2 for a detailed explanation of mediation). Mediation-adjusted models for all three datasets and the combined data show age-associated shortening rates consistent with longitudinal studies (40.19 bp/year in the combined data) and larger FAB effects (38.62 bp/year) than previously reported (Table2). PBY effects were less pronounced and more heterogeneous compared to linear regression models. Both the UK Twins and the NHLBI-FHS dataset showed negative correlations between PBY and telomere length, while the Asklepios dataset indicated a positive correlation between PBY and telomere length (Fig.3). Sobel tests were performed on each mediation interaction to test for statistical significance (Table S4), and all interactions’ z-scores indicated P-values < 0.05.
Fig 2.
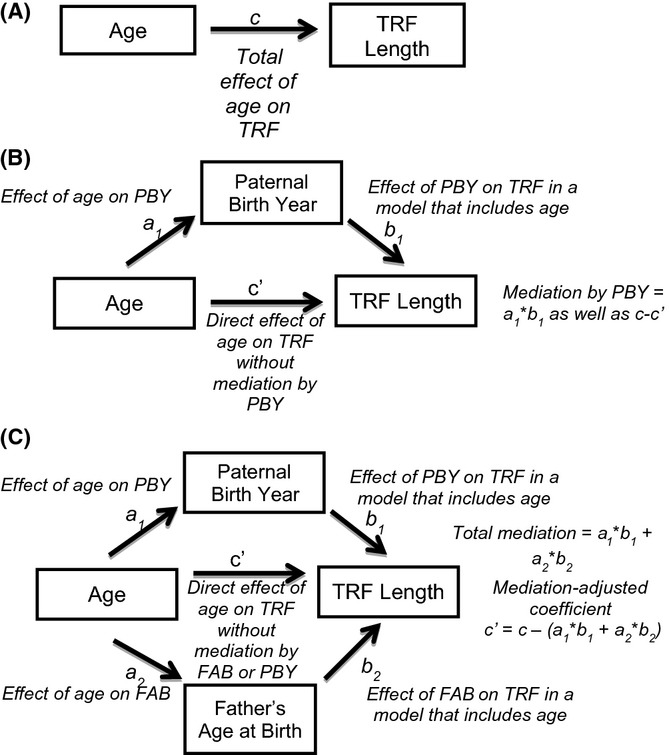
Method by which mediation analysis accounts for variable collinearity. Mediation analysis seeks to determine how much of an independent variable’s effect on a dependent variable is influenced by an interaction through a third variable, the mediator.
Table 2.
Mediation-adjusted models
| Independent variable | Mediation (bp/year) | Total mediation | Adjusted coefficient | ||
|---|---|---|---|---|---|
| Age | PBY | FAB | |||
| UK Twins | |||||
| Age | N/A | 20.26 | 0.70 | 20.95 | −41.64 |
| PBY | 31.58 | N/A | −7.10 | 24.47 | −12.38 |
| FAB | −4.50 | −23.19 | N/A | −27.69 | 36.58 |
| NHLBI-FHS | |||||
| Age | N/A | 15.12 | 0.39 | 15.51 | −36.81 |
| PBY | 27.47 | N/A | −8.72 | 18.75 | −5.94 |
| FAB | −1.90 | −23.41 | N/A | −25.31 | 37.97 |
| Asklepios | |||||
| Age | N/A | 20.12 | 2.37 | 22.49 | −48.42 |
| PBY | 20.82 | N/A | −24.02 | −3.20 | 6.29 |
| FAB | −14.08 | −41.06 | N/A | −55.14 | 58.23 |
| Combined | |||||
| Age | N/A | 18.00 | 0.74 | 18.75 | −40.19 |
| PBY | 28.10 | N/A | −10.04 | 18.06 | −7.18 |
| FAB | −3.05 | −23.77 | N/A | −26.82 | 38.62 |
The effect of each independent variable (age, PBY, and FAB) is adjusted for the effects of each other independent variable on telomere length and their collinearity within each dataset. The adjusted coefficients (far right) illustrate age-associated telomere shortening rates consistent with longitudinal observations, heterogeneity in PBY effects and reveal larger FAB effects than previously reported.
Fig 3.
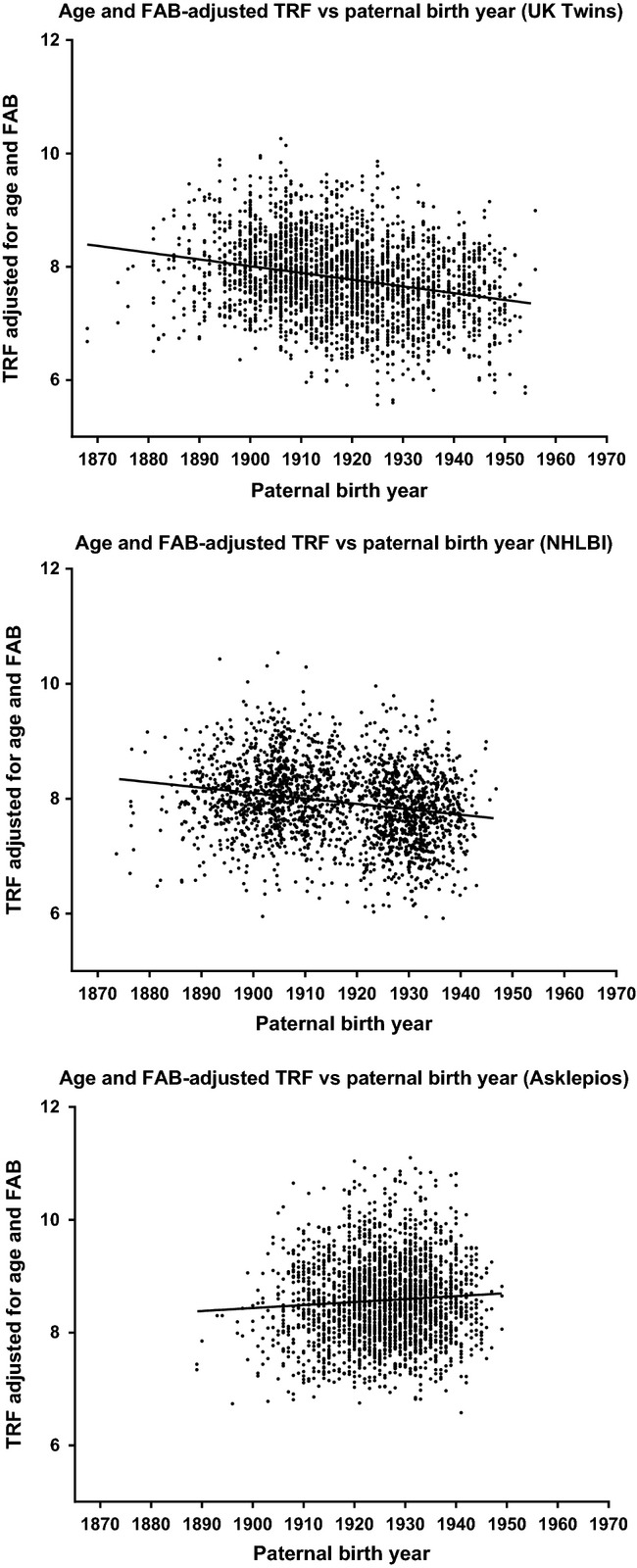
Paternal birth year effect in all three study populations. Age- and FAB-corrected telomere lengths demonstrate the paternal birth year effect in all three study populations.
Segregating by offspring gender and repeating the mediation analysis revealed the same trends as considering the entire population as a whole (Tables S5 and S6) in all datasets with the exception of the UK Twins data, where a very small number of males (N = 199 males, 2511 females) complicate interpretation.
Pseudo-longitudinal shortening rate in the UK Twins data is more consistent with mediation-adjusted age-associated shortening
The UK Twins dataset can be used to obtain another measurement of age-associated telomere shortening rate because not all twin pairs’ telomeres were measured at the same time. The difference in telomere length between twins in each twin pair is centered around zero for twins measured at the same time; however, as the time between visits increases, the difference between the first twin measured and the second twin measured increases due to age-associated telomere shortening. Because the twin measured second is older at the time of measurement than the twin measured first, this divergence in telomere length is due in part to age-associated telomere shortening. Although each measurement is a different individual, the mean intertwin variation is zero except for the effects of age and any variables that may contribute to delayed sampling of the second twin, such as socioeconomic status, illness, or travel. The difference in telomere length between the first twin and the second twin can therefore be viewed as a ‘pseudo-longitudinal’ telomere shortening assay. This pseudo-longitudinal shortening assay may give a more accurate picture of age-associated telomere shortening rate compared to a simple cross-sectional analysis because twins share both FAB and PBY.
Figure4 shows the difference in telomere length between the first twin measured and second twin measured for all twin pairs with different sample collection dates as a function of time elapsed between sample collection for the first and second twin measured. This indicates a telomere shortening rate of 62.13 bp/year. This shortening rate is substantially higher than the rate produced from the mediation-adjusted model, although that rate (gray line in Fig.4) falls within the 95% confidence interval for the pseudo-longitudinal regression.
Fig 4.
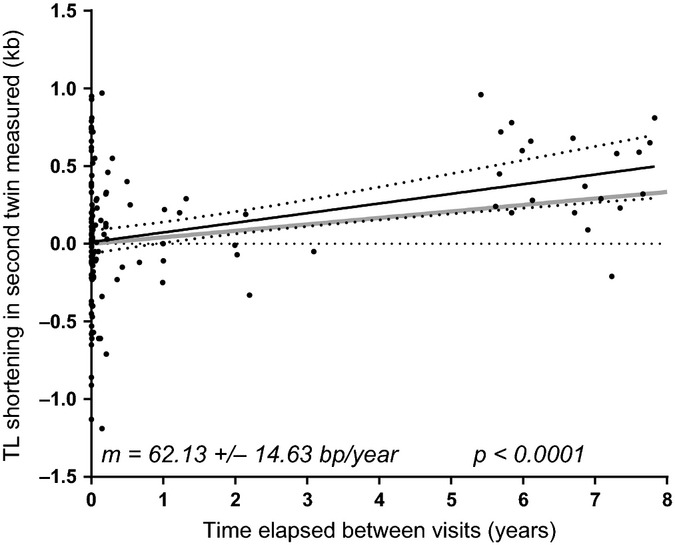
Pseudo-longitudinal measurements of telomere shortening in the UK Twins cohort. Because the difference in telomere length between the first and second twin measured is zero on average in twins measured at nearly the same time, the measurement of twins years apart may be considered a better measurement of telomere shortening rate. Pseudo-longitudinal telomere shortening in the UK Twins cohort is more consistent with the mediation-adjusted model (gray line) than simple cross-sectional analysis. The 95% confidence interval of the pseudo-longitudinal regression is demarcated by dotted lines.
Correcting telomere length for age and paternal effects reveals a secular trend in initial telomere length
Correcting telomere length for the mediation-adjusted effects of age, PBY, and FAB in each dataset, a calculation of initial telomere length after removing the effects of the paternal variables, reveals a time-dependent trend toward decreasing telomere length in relation to date of birth of 7.24, 14.97, and 23.80 bp/year in the UK Twins, NHLBI, and Asklepios data, respectively (Fig.5). As the mediation-adjusted effects account for the collinearity of the variables, the presence of a trend after correcting for these effects indicates the presence of at least one missing variable that impacts telomere length and has some relationship with time. As this trend was not accounted for by the PBY effect, this may represent the feed-forward effect predicted by the dual inheritance modality of telomere length or a relatively recent (occurring in the 1900s) environmental exposure affecting initial birth telomere length.
Fig 5.
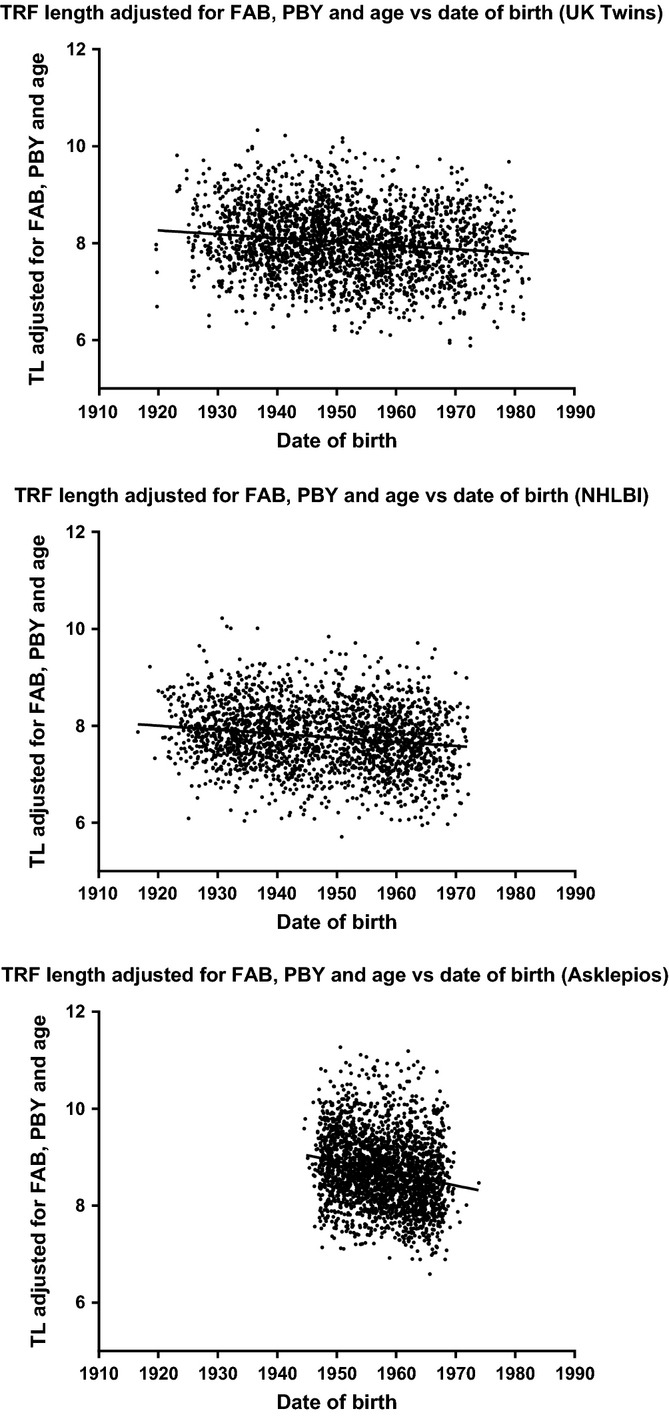
Changes in initial telomere length over time. Telomere length displays a negative correlation with date of birth after correcting for the mediation-adjusted effects of age, PBY, and FAB, showing that time-dependent influences on telomere length remain to be discovered.
Discussion
Because different species (Gomes et al., 2011) and human ethnic groups possess variable telomere lengths, average initial telomere length must be capable of change over evolutionary time. However, it was unknown whether a secular trend in initial telomere length was currently present in human populations and, if so, what may be driving such a change. We initially hypothesized that the FAB effect and the observed increase in sperm TL in older men may have been a marker for such a secular trend in initial telomere length, as both observations could have resulted from a decrease in initial telomere length over time. Our analysis revealed that although initial telomere length does appear to be changing in relation to PBY, the FAB effect becomes larger after accounting for the collinearity between FAB, PBY, and age. The mechanism for the progressive telomere lengthening in sperm cell progenitors that may be driving this FAB effect is a very interesting enigma, as an indefinite upward trend in telomere length has not been observed in telomerase-positive cells in tissue culture.
It is important to note that PBY is used here as a proxy variable for some unknown variable that has a relationship with time, not to indicate that PBY itself is driving such a change. The PBY effect appears to be highly variable between populations, and it is possible that this represents sensitivity to past differences in the environment, for instance in exposure to industrial pollution or malnutrition. Both datasets showing shortening in response to PBY were composed of heterogeneous, predominantly urban populations, while the population of the Asklepios study was composed of individuals living in two smaller communities in Belgium; it is possible that some urban environmental exposure may be driving this difference. Although technical differences in telomere length measurement may partially explain differences in mean telomere length between populations under study, the Asklepios cohort exhibited longer telomeres than both of the other datasets even after accounting for the study-specific effects of age, PBY, and FAB, suggesting that the difference in PBY effect may have existed for some time and this trend may explain why the Asklepios population has longer telomeres. It is also possible that some feature of sample selection in each study may be responsible for the difference in PBY effect, as the Asklepios population only included individuals that were free of overt cardiovascular disease while both of the other datasets did not exclude individuals based on their health, potentially biasing the trend observed in that cohort. The variability of the PBY effect may be one of the factors driving heterogeneity in telomere length between human groups, and the magnitude of this effect is large enough that it could yield relatively large changes in LTL over relatively short periods of time. For example, if the UK Twins population and Asklepios population started at the same telomere length and diverged only according to their PBY effect over time, they would be separated by 1 kb in mean telomere length in 53.5 years. It is likely that the PBY effect will vary both spatially and chronologically due to as-yet-undiscovered genetic, environmental, historical, and demographic drivers.
Particularly in the developed world, the prevalence of many environmental factors known to impact telomere length, such as those derived from traffic pollution and urban garbage (Hoxha et al., 2009; De Felice et al., 2012; Hou et al., 2012), changed dramatically in the past 200 years due to the industrial revolution, and this change may manifest as a change in telomere length at birth if those environmental changes alter telomere length in germ cells or during fetal development.
Accounting for the collinearity between the variables in the model and including PBY resolves the discrepancy in measured age-associated telomere shortening rate between cross-sectional and longitudinal studies, as the inclusion of both PBY and FAB in the model produces telomere shortening rates consistent with longitudinal studies in all three of these cross-sectional datasets. Many cross-sectional studies have reported heterogeneity in the effects of age and FAB, and it is possible that re-analysis of their data using this method accounting for PBY and the collinearity of these variables would clarify these relationships. The true heterogeneity in these effects remains poorly understood at this juncture because very few longitudinal studies have been conducted and most cross-sectional studies analyzed each effect without accounting for collinearity with other variables.
A secular trend, a negative relationship between initial telomere length and date of birth, exists in all three datasets even after removing the effects of age, FAB, and PBY, demonstrating that at least one additional variable is causing a reduction in initial telomere length over time. A maternal effect is one possibility, but it was not possible in the current study to evaluate maternal variables and paternal variables at the same time. If the paternal effects have existed for more than one generation (which is the case in at least one known population (Eisenberg et al., 2012)), maternal effects on telomere length are a logical consequence of the heritability of telomere length, as women would inherit altered telomere length from their fathers and pass this different initial telomere length to their offspring through their germ line. In addition to a feed-forward effect from paternally inherited shorter telomeres, maternal effects may be driven through environmental stresses to oocytes arrested in meiosis for decades that could damage telomeres and drive telomere shortening with advanced maternal age. There is some evidence that paternally transmitted telomeres are more important for determining telomere length in the offspring (Nordfjall et al., 2010), and inheriting mutant telomerase alleles paternally is more detrimental to telomere length compared to the same alleles transmitted maternally (Diaz de Leon et al., 2010), although the parent-specific heritability of telomere length is still not fully determined (Eisenberg, 2014). A maternal effect has not been definitively ruled out in the general population and at least some amount of telomere length is inherited maternally, as wild-type offspring of female TERT mutant heterozygotes inherit short telomeres from their mother, albeit to a lesser degree (Diaz de Leon et al., 2010).
The epigenetic aspects of telomere length heritability may be largely driven by male gametes (De Meyer & Eisenberg, 2014) because egg progenitor cells undergo far fewer divisions per generation compared to sperm progenitor cells that divide throughout the lifetime of a male. However, oocytes remain in meiotic arrest for decades and could also be subjected to environmental stressors that could result in shortening of telomeres in the absence of cell division via breakage. Because half the genome is inherited from the father, the FAB effect should be roughly half of the lengthening in sperm telomeres; the FAB effect reported in this model is substantially larger than half of the reported lengthening in sperm (57 bp/year). However, the measurement of lengthening in sperm may have been subject to the same biases of other cross-sectional analyses, for example not accounting for the collinearity in the sperm donors between age and important variables known to impact telomere length, such as FAB, nor for the previously unreported PBY effect. It is possible that the actual lengthening in sperm progenitor cells over time is substantially smaller than reported previously because the PBY and FAB trends observed in this analysis that reduce observed age-associated shortening should exacerbate cross-sectionally the observed lengthening of sperm telomere length. Longitudinal analysis of telomere dynamics in sperm should resolve the discrepancy, as a longitudinal analysis would not be subject to underlying shifts in initial telomere length. It is also possible that sperm telomeres are more important than oocyte telomeres in determining offspring telomere length, and the FAB effect is more than half of the lengthening observed in sperm as a result of this difference.
The causes of the PBY effect and secular trend in initial telomere length must be relatively new, as these trends would have pushed these populations into potentially pathological telomere length ranges, those observed in families with telomeropathies, 2–3 kb shorter (Alter et al., 2012), in a few centuries. Given that many of the environmental insults known to impact telomeres are the results of industrial pollution, the PBY effect and the secular trend in initial telomere length may be the cumulative result of many different evolutionarily novel insults to telomere maintenance. The presence of secular trends in many other parameters, such as obesity, height, pubertal onset, and smoking behavior may also be related to the secular trend in initial telomere length. However, many of these trends display substantial nonlinearity that is not observed in the secular trend in initial telomere length, suggesting the other secular trends may not be driving the change in telomere length. At the present time, it is unclear how long the observed trend in shorter initial telomere length has been occurring, although measurement of telomere length in archeological samples may be able to provide insight.
While the biological significance of the secular trend in reduced initial telomere length is not yet understood, there may be implications for public health. Correlations exist between short telomere length and a wide variety of pathologies; for example, robust inverse correlations exist between telomere length and asthma incidence and severity (Albrecht et al., 2014), and the incidence of asthma in the developed world has been rising precipitously in the recent past (Eder et al., 2006). Although it is not known whether the relationship between asthma and telomere length is causative or correlative, if it were partially causative, a decrease in initial telomere length in the population could increase asthma risk and may represent one cause for the increase in asthma incidence. Lungs seem to be one of the organs most sensitive to short telomere length and impaired telomere maintenance, with diseases such as idiopathic pulmonary fibrosis (IPF) and other lung pathology in the earliest generations of families with mutations in TERT (Diaz de Leon et al., 2011). Furthermore, short telomeres are protective for certain cancers and risk factors for other types of cancers (Wentzensen et al., 2011; Anic et al., 2013; Walcott et al., 2013), and a secular trend in initial telomere length may impact the frequencies of these cancers.
In conclusion, we have identified a change in initial telomere length linked to paternal birth year. In addition, we have shown how inclusion of PBY in the analysis of cross-sectional telomere datasets can reconcile the differences between cross-sectional and longitudinal measurements of telomere shortening. This reconciliation has refined our understanding of the age-associated rate of telomere shortening, and we presented evidence that longitudinal measurements are more likely to be correct. We have also shown that the effect of the father’s age at birth of their offspring on their offspring’s telomere length is larger than previously reported after accounting for FAB’s collinearity with other variables. Lastly, we have identified a downward secular trend in initial telomere length after accounting for the known age and paternal effects on telomere length that may have public health implications, which indicates that other influences on telomere length remain to be discovered.
Experimental procedures
Mediation analysis
The multilevel R package (Bliese, 2006) was used to perform the mediation analysis, and results were confirmed via the method of Preacher and Hayes (Preacher & Hayes, 2008). Mediation analysis is designed to disentangle the effects of collinear variables and allows quantitation of the amount of a given independent variable’s total effect on a dependent variable that is exerted via an indirect effect through at least one other variable, the mediator. Using the product-of-coefficients approach (Preacher & Hayes, 2008), the total effect of each independent variable (age, PBY, and FAB) on the dependent variable (TRF length) is determined by linear regression analysis independently (Fig.2A). The effect of each independent variable on each other independent variable is then determined, as are the effects of each independent variable on telomere (TRF) length in regression models that include one other independent variable (regression matrices are given in Table S2). The indirect effect of the independent variable through the mediator is then computed as the product of the independent variable’s effect on the mediator (a1) and the mediator’s effect on the dependent variable accounting for the independent variable (b1), which will also correspond to the difference between the independent variable’s effect on the dependent variable (c) and the independent variable’s effect on the dependent variable in a model that includes the mediator (c’, Fig.2B). This procedure can be carried out for any number of mediators to obtain an estimate of the direct effect of an independent variable accounting for its collinearity with mediator variables (Fig.2C).
Statistics
Datasets were obtained from the groups that initially collected the samples and measured telomere length (Higgins et al., 1996; Andrew et al., 2001; De Meyer et al., 2007; Rietzschel et al., 2007; Kimura et al., 2008). All three datasets used the TRF assay Southern blot-based telomere measurement technique, facilitating interpopulation comparisons. Linear regressions and Pearson’s correlations were computed using the minitab 17 software package.
Age- and paternal effects-corrected telomere lengths used for Figures3 and 5 were computed by adjusting all telomere lengths using the mediation-adjusted coefficients from this work to age and father’s age at birth = 0 and paternal birth year = 1900.
Acknowledgments
The authors would like to thank Dr. Abraham Aviv for helpful discussions and facilitating collaboration. The Asklepios study thanks the communities, residents, and general practitioners of Erpe-Mere and Nieuwerkerken for their invaluable efforts in making the study possible.
Funding
The TwinsUK study was funded by the Wellcome Trust; European Community’s Seventh Framework Programme (FP7/2007-2013). The study also receives support from the National Institute for Health Research (NIHR) BioResource Clinical Research Facility and Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The NHLBI Family Heart Study was funded by National Heart, Lung, and Blood Institute (U01 HL56563, U01 HL56564, U01 HL56565, U01 HL56566, U01 HL56567, U01 HL56568, and U01 HL56569). The Asklepios study is supported by FWO-Flanders research grants G.0427.03 and G.0838.10N. These studies were supported in part by the Simmons Cancer Center Support Grant 5P30 CA142543 and support from the Southland Financial Corporation Distinguished Chair in Geriatric Research. This work was performed in space constructed with support from National Institute of Health Grant C06 RR30414.
Conflicts of interest
The authors have no conflicts of interest to declare.
Author contributions
B.H. and K.B. performed experimental analysis. B.H., T.D.M., W.E.W., and J.W.S. contributed to the design and concepts of the study. B.H. wrote the manuscript. T.D.M., M.M., S.C.H., T.D.S., and J.W.S. edited the manuscript. T.D.M., M.M., S.C.H., S.B., M.L.D.B., E.R.R., and T.D.S. provided data for the study.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site.
Figure S1. Multicollinearity between FAB, PBY and Age.
Table S1. Comparison of cross-sectional and longitudinal measurements of telomere shortening.
Table S2. Multicollinearity between FAB, PBY, MAB, MBY and Age in the combined datasets.
Table S3. Regression matrices for mediation analysis.
Table S4. Sobel tests for mediation.
Table S5. Gender-segregated mediation analysis.
Table S6. Sobel tests for gender-segregated mediation analysis.
Video S1. Illustration of how changing initial telomere length would bias cross-sectional telomere shortening rates. Cross-sectional analyses rely on the assumption that initial telomere length is not changing. If initial length is changing, observed shortening rates are biased in the direction opposite the change in initial telomere length, as the change in initial length is incorrectly ascribed to aging. The video shows birth and aging of individuals (blue circles) over time. Individuals are born at age zero and move to the right as they age; other individuals are born at later times and at differing initial telomere lengths, but all individuals shorten at the same rate over time. In the first model, initial telomere length does not change and the cross-sectional analysis, conducted when the solid line is drawn, yields a correct measurement of telomere shortening rate. Initial telomere length is increasing in the second model, which results in overestimation of the telomere shortening rate (solid line) at analysis compared with the actual shortening rate (dashed line). The third model shows how reduction in initial telomere length over time would yield an underestimate of telomere shortening rate at cross-sectional analysis.
References
- Albrecht E, Sillanpaa E, Karrasch S, Alves AC, Codd V, Hovatta I, Buxton JL, Nelson CP, Broer L, Hagg S, Mangino M, Willemsen G, Surakka I, Ferreira MA, Amin N, Oostra BA, Backmand HM, Peltonen M, Sarna S, Rantanen T, Sipila S, Korhonen T, Madden PA, Gieger C, Jorres RA, Heinrich J, Behr J, Huber RM, Peters A, Strauch K, Wichmann HE, Waldenberger M, Blakemore AI, de Geus EJ, Nyholt DR, Henders AK, Piirila PL, Rissanen A, Magnusson PK, Vinuela A, Pietilainen KH, Martin NG, Pedersen NL, Boomsma DI, Spector TD, van Duijn CM, Kaprio J, Samani NJ, Jarvelin MR, Schulz H. Telomere length in circulating leukocytes is associated with lung function and disease. The European Respiratory Journal. 2014;43:983–992. doi: 10.1183/09031936.00046213. [DOI] [PubMed] [Google Scholar]
- Alter BP, Rosenberg PS, Giri N, Baerlocher GM, Lansdorp PM, Savage SA. Telomere length is associated with disease severity and declines with age in dyskeratosis congenita. Haematologica. 2012;97:353–359. doi: 10.3324/haematol.2011.055269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew T, Hart DJ, Snieder H, de Lange M, Spector TD, MacGregor AJ. Are twins and singletons comparable? A study of disease-related and lifestyle characteristics in adult women. Twin Research. 2001;4:464–477. doi: 10.1375/1369052012803. [DOI] [PubMed] [Google Scholar]
- Anic GM, Sondak VK, Messina JL, Fenske NA, Zager JS, Cherpelis BS, Lee JH, Fulp WJ, Epling-Burnette PK, Park JY, Rollison DE. Telomere length and risk of melanoma, squamous cell carcinoma, and basal cell carcinoma. Cancer Epidemiol. 2013;37:434–439. doi: 10.1016/j.canep.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanios M, Chen JL, Chang YP, Brodsky RA, Hawkins A, Griffin CA, Eshleman JR, Cohen AR, Chakravarti A, Hamosh A, Greider CW. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc. Natl Acad. Sci. USA. 2005;102:15960–15964. doi: 10.1073/pnas.0508124102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston KI, Hunt SC, Susser E, Kimura M, Factor-Litvak P, Carrell D, Aviv A. Divergence of sperm and leukocyte age-dependent telomere dynamics: implications for male-driven evolution of telomere length in humans. Mol. Hum. Reprod. 2012;18:517–522. doi: 10.1093/molehr/gas028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A, Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, Srinivasan SR, Berenson GS. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am. J. Epidemiol. 2009;169:323–329. doi: 10.1093/aje/kwn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliese P. 2006. Multilevel Modeling in R (2.2)–A Brief Introduction to R, the multilevel package and the nlme packageed eds): October.
- Bojesen SE. Telomeres and human health. J. Intern. Med. 2013;274:399–413. doi: 10.1111/joim.12083. [DOI] [PubMed] [Google Scholar]
- Bray I, Gunnell D, Davey Smith G. Advanced paternal age: how old is too old? J. Epidemiol. Community Health. 2006;60:851–853. doi: 10.1136/jech.2005.045179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Kimura M, Kim S, Cao X, Srinivasan SR, Berenson GS, Kark JD, Aviv A. Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: age-dependent telomere shortening is the rule. The Journals of Gerontology. Series A, Biological sciences and medical sciences. 2011;66:312–319. doi: 10.1093/gerona/glq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, Greider CW, DePinho RA. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell. 1999;97:527–538. doi: 10.1016/s0092-8674(00)80762-x. [DOI] [PubMed] [Google Scholar]
- Daniali L, Benetos A, Susser E, Kark JD, Labat C, Kimura M, Desai K, Granick M, Aviv A. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat. Commun. 2013;4:1597. doi: 10.1038/ncomms2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice B, Nappi C, Zizolfi B, Guida M, Di Spiezio SA, Bifulco G, Guida M. Telomere shortening in women resident close to waste landfill sites. Gene. 2012;500:101–106. doi: 10.1016/j.gene.2012.03.040. [DOI] [PubMed] [Google Scholar]
- De Meyer T, Eisenberg DT. Possible technical and biological explanations for the ‘parental telomere length inheritance discrepancy’ enigma. European Journal of Human Genetics. 2014:3–4. doi: 10.1038/ejhg.2014.65. EJHG. 23, [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyer T, Rietzschel ER, De BM, De Bacquer D, Van Criekinge W, De Backer GG, Gillebert TC, Van Oostveldt P, Bekaert S, Asklepios I. Paternal age at birth is an important determinant of offspring telomere length. Hum. Mol. Genet. 2007;16:3097–3102. doi: 10.1093/hmg/ddm271. [DOI] [PubMed] [Google Scholar]
- De Meyer T, Vandepitte K, Denil S, De Buyzere ML, Rietzschel ER, Bekaert S. A non-genetic, epigenetic-like mechanism of telomere length inheritance? European Journal of Human Genetics. 2014;22:10–11. doi: 10.1038/ejhg.2013.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz de Leon A, Cronkhite JT, Katzenstein AL, Godwin JD, Raghu G, Glazer CS, Rosenblatt RL, Girod CE, Garrity ER, Xing C, Garcia CK. Telomere lengths, pulmonary fibrosis and telomerase (TERT) mutations. PLoS ONE. 2010;5:e10680. doi: 10.1371/journal.pone.0010680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz de Leon A, Cronkhite JT, Yilmaz C, Brewington C, Wang R, Xing C, Hsia CC, Garcia CK. Subclinical lung disease, macrocytosis, and premature graying in kindreds with telomerase (TERT) mutations. Chest. 2011;140:753–763. doi: 10.1378/chest.10-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder W, Ege MJ, von Mutius E. The asthma epidemic. The New England Journal of Medicine. 2006;355:2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- Ehrlenbach S, Willeit P, Kiechl S, Willeit J, Reindl M, Schanda K, Kronenberg F, Brandstatter A. Influences on the reduction of relative telomere length over 10 years in the population-based Bruneck Study: introduction of a well-controlled high-throughput assay. Int. J. Epidemiol. 2009;38:1725–1734. doi: 10.1093/ije/dyp273. [DOI] [PubMed] [Google Scholar]
- Eisenberg DT. Inconsistent inheritance of telomere length (TL): is offspring TL more strongly correlated with maternal or paternal TL? European Journal of Human Genetics. 2014;22:8–9. doi: 10.1038/ejhg.2013.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DT, Salpea KD, Kuzawa CW, Hayes MG, Humphries SE European Atherosclerosis Research Study II Group. Substantial variation in qPCR measured mean blood telomere lengths in young men from eleven European countries. American Journal of Human Biology. 2011;23:228–231. doi: 10.1002/ajhb.21126. [DOI] [PubMed] [Google Scholar]
- Eisenberg DT, Hayes MG, Kuzawa CW. Delayed paternal age of reproduction in humans is associated with longer telomeres across two generations of descendants. Proc. Natl Acad. Sci. USA. 2012;109:10251–10256. doi: 10.1073/pnas.1202092109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzaneh-Far R, Lin J, Epel E, Lapham K, Blackburn E, Whooley MA. Telomere length trajectory and its determinants in persons with coronary artery disease: longitudinal findings from the heart and soul study. PLoS ONE. 2010;5:e8612. doi: 10.1371/journal.pone.0008612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JP, Li S, Srinivasan SR, Chen W, Kimura M, Lu X, Berenson GS, Aviv A. Rise in insulin resistance is associated with escalated telomere attrition. Circulation. 2005;111:2171–2177. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
- Gomes NM, Ryder OA, Houck ML, Charter SJ, Walker W, Forsyth NR, Austad SN, Venditti C, Pagel M, Shay JW, Wright WE. Comparative biology of mammalian telomeres: hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell. 2011;10:761–768. doi: 10.1111/j.1474-9726.2011.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M, Province M, Heiss G, Eckfeldt J, Ellison RC, Folsom AR, Rao DC, Sprafka JM, Williams R. NHLBI Family Heart Study: objectives and design. Am. J. Epidemiol. 1996;143:1219–1228. [Google Scholar]
- Holohan B, Wright WE, Shay JW. Cell biology of disease: telomeropathies: an emerging spectrum disorder. J. Cell. Biol. 2014;205:289–299. doi: 10.1083/jcb.201401012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Wang S, Dou C, Zhang X, Yu Y, Zheng Y, Avula U, Hoxha M, Diaz A, McCracken J, Barretta F, Marinelli B, Bertazzi PA, Schwartz J, Baccarelli AA. Air pollution exposure and telomere length in highly exposed subjects in Beijing, China: a repeated-measure study. Environ. Int. 2012;48:71–77. doi: 10.1016/j.envint.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben JM, Giltay EJ, Rius-Ottenheim N, Hageman GJ, Kromhout D. Telomere length and mortality in elderly men: the Zutphen Elderly Study. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2011;66:38–44. doi: 10.1093/gerona/glq164. [DOI] [PubMed] [Google Scholar]
- Hoxha M, Dioni L, Bonzini M, Pesatori AC, Fustinoni S, Cavallo D, Carugno M, Albetti B, Marinelli B, Schwartz J, Bertazzi PA, Baccarelli A. Association between leukocyte telomere shortening and exposure to traffic pollution: a cross-sectional study on traffic officers and indoor office workers. Environmental Health. 2009;8:41. doi: 10.1186/1476-069X-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Cherkas LF, Kato BS, Demissie S, Hjelmborg JB, Brimacombe M, Cupples A, Hunkin JL, Gardner JP, Lu X, Cao X, Sastrasinh M, Province MA, Hunt SC, Christensen K, Levy D, Spector TD, Aviv A. Offspring’s leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet. 2008;4:e37. doi: 10.1371/journal.pgen.0040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komlos J, Breitfelder A. Height of US-born non-Hispanic children and adolescents ages 2-19, born 1942-2002 in the NHANES samples. American Journal of Human Biology. 2008;20:66–71. doi: 10.1002/ajhb.20677. [DOI] [PubMed] [Google Scholar]
- de Lange T. How shelterin solves the telomere end-protection problem. Cold Spring Harb. Symp. Quant. Biol. 2010;75:167–177. doi: 10.1101/sqb.2010.75.017. [DOI] [PubMed] [Google Scholar]
- Nordfjall K, Svenson U, Norrback KF, Adolfsson R, Roos G. Large-scale parent-child comparison confirms a strong paternal influence on telomere length. European Journal of Human Genetics. 2010;18:385–389. doi: 10.1038/ejhg.2009.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Rietzschel ER, De Buyzere ML, Bekaert S, Segers P, De Bacquer D, Cooman L, Van Damme P, Cassiman P, Langlois M, van Oostveldt P, Verdonck P, De Backer G, Gillebert TC, Asklepios I. Rationale, design, methods and baseline characteristics of the Asklepios Study. European Journal of Cardiovascular Prevention and Rehabilitation. 2007;14:179–191. doi: 10.1097/HJR.0b013e328012c380. [DOI] [PubMed] [Google Scholar]
- Romero CX, Romero TE, Shlay JC, Ogden LG, Dabelea D. Changing trends in the prevalence and disparities of obesity and other cardiovascular disease risk factors in three racial/ethnic groups of USA adults. Advances in Preventive Medicine. 2012;2012:172423. doi: 10.1155/2012/172423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppari J, Juul A. Trends in puberty timing in humans and environmental modifiers. Mol. Cell. Endocrinol. 2010;324:39–44. doi: 10.1016/j.mce.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Unryn BM, Cook LS, Riabowol KT. Paternal age is positively linked to telomere length of children. Aging Cell. 2005;4:97–101. doi: 10.1111/j.1474-9728.2005.00144.x. [DOI] [PubMed] [Google Scholar]
- Walcott F, Rajaraman P, Gadalla SM, Inskip PD, Purdue MP, Albanes D, Orr E, De Vivo I, Savage SA. Telomere length and risk of glioma. Cancer Epidemiol. 2013;37:935–938. doi: 10.1016/j.canep.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA. The association of telomere length and cancer: a meta-analysis. Cancer Epidemiology, Biomarkers & Prevention. 2011;20:1238–1250. doi: 10.1158/1055-9965.EPI-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev. Genet. 1996;18:173–179. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Multicollinearity between FAB, PBY and Age.
Table S1. Comparison of cross-sectional and longitudinal measurements of telomere shortening.
Table S2. Multicollinearity between FAB, PBY, MAB, MBY and Age in the combined datasets.
Table S3. Regression matrices for mediation analysis.
Table S4. Sobel tests for mediation.
Table S5. Gender-segregated mediation analysis.
Table S6. Sobel tests for gender-segregated mediation analysis.
Video S1. Illustration of how changing initial telomere length would bias cross-sectional telomere shortening rates. Cross-sectional analyses rely on the assumption that initial telomere length is not changing. If initial length is changing, observed shortening rates are biased in the direction opposite the change in initial telomere length, as the change in initial length is incorrectly ascribed to aging. The video shows birth and aging of individuals (blue circles) over time. Individuals are born at age zero and move to the right as they age; other individuals are born at later times and at differing initial telomere lengths, but all individuals shorten at the same rate over time. In the first model, initial telomere length does not change and the cross-sectional analysis, conducted when the solid line is drawn, yields a correct measurement of telomere shortening rate. Initial telomere length is increasing in the second model, which results in overestimation of the telomere shortening rate (solid line) at analysis compared with the actual shortening rate (dashed line). The third model shows how reduction in initial telomere length over time would yield an underestimate of telomere shortening rate at cross-sectional analysis.


