Abstract
Background
Guidelines specify a definition of chronic obstructive pulmonary disease (COPD) as a post-bronchodilator ratio of one-second forced expiratory volume (FEV1) to forced vital capacity (FVC) of less than70%. Some investigators apply this type of “fixed ratio” criterion while more recently others employ a threshold based on the age-sex specific predicted lower limit of normal (LLN). Further studies still report prevalences based on pre-bronchodilator spirometry. The impact of these different methods on prevalence estimates is poorly understood and makes an informative review of the available prevalence surveys impossible.
Methods
Pre- and post-bronchodilator spirometry results were taken from the Burden of Obstructive Lung Disease (BOLD) survey in 16 centres. Using a post-bronchodilator ratio less than LLN as our gold standard, we calculated simple multiplicative adjustments to calibrate other reported prevalence estimates to gold standard equivalent estimates. These adjustments were then tested on independent data sets from six further BOLD centres and five PLATINO study centres.
Results
Prevalence estimates based on pre-bronchodilator fixed ratio measurements were 5-25% higher than the directly measured value and were strongly positively biased with age and prevalence level. Applying simple multiplicative adjustments gave prevalence estimates that were almost unbiased and within 5% of the directly measured result.
Conclusions
Using the BOLD data we have been able to estimate COPD prevalences based on post-bronchodilator FEV1/FVC<LLN by adjusting estimates based on other common definitions. This makes possible wider and more meaningful comparisons of available published findings.
Introduction
There is good reason to believe that chronic obstructive pulmonary disease (COPD) is a common cause of death and disability world-wide. (1)Nevertheless there is relatively little direct evidence in relation to its prevalence globally. This is in part because the condition is defined by spirometry and spirometric surveys of general populations have been uncommon. (2)However, variation in the methods used to assess the prevalence of disease, when such surveys have been undertaken, has also seriously reduced the availability of data on prevalence based on standard definitions.
Measurement of chronic airflow obstruction (CAO) is central to the diagnosis of chronic obstructive pulmonary disease (COPD) where CAO is defined in this context as a low ratio of one-second forced expiratory volume (FEV1) to forced vital capacity (FVC) after inhaling a bronchodilator. However, the definition of “low” varies. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) has provided a definition of COPD as a post-bronchodilator FEV1/FVC ratio of less than 70%, where bronchodilation using inhalation of 400μg albuterol (salbutamol) is suggested. (3)Because the FEV1/FVC ratio is strongly dependent on age, an argument has been made for defining COPD as a ratio below the lower limit of normal (LLN) instead. (4) The use of these different criteria, and the practice in many cases of not using a bronchodilator, complicate meaningful comparisons of reported COPD prevalences in the literature.
Conversion of prevalence estimates made using one set of spirometric criteria to those that would be expected if an alternative set of spirometric criteria had been applied by the computation of adjustment factors would allow the comparison of studies that had used different criteria. In this study we have used data from 16 BOLD surveys to estimate the adjustment factors and have corroborated these using data from six more recently completed BOLD surveys, as well as the five PLATINO surveys.
Methods
Datasets
Both the BOLD (5,6) and PLATINO (7) studies are international studies of the burden of COPD which use similar protocols to assess pre- and post-bronchodilator spirometry in populations aged 40 years old and over. The estimation of the adjustment factors are based on results from the first 16 BOLD centres in 15 countries using the same study protocol but covering a range of incomes and health service models (see Table 1). Data from a further six BOLD centres as well as the PLATINO (7) sites, in Latin America, were used to corroborate the results. In all centres bronchodilator spirometry was measured before and between 15 and 60 minutes after 200μg salbutamol administered by a spacer.
Table 1.
Directly observed prevalence of COA in 16 BOLD sites using different definitions of COPD.
| BOLD site | Post-bronchodilator FEV1/FVC<LLN | Post-bronchodilator FEV1/FVC<70% | Pre-bronchodilator FEV1/FVC<70% | |||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Sydney, Australia | 6.1 (1.6) | 13.9 (2.2) | 18.5 (2.5) | 19.6 (2.6) | 27.0 (2.9) | 22.8 (2.7) |
| Reykyavik, Iceland | 9.3 (1.5) | 13.7 (1.9) | 18.2 (2.0) | 17.4 (2.1) | 31.5 (2.3) | 27.6 (2.5) |
| Lisbon, Portugal | 9.3 (2.4) | 7.5 (1.2) | 18.7 (3.4) | 10.7 (1.6) | 21.2 (3.8) | 17.5 (1.3) |
| Hannover, Germany | 9.8 (1.7) | 7.0 (1.5) | 18.3 (2.3) | 9.6 (1.7) | 26.9 (2.6) | 15.5 (2.2) |
| Guangzhou, China | 10.5 (2.1) | 7.0 (1.9) | 15.3 (2.5) | 8.5 (2.0) | 18.2 (2.6) | 12.6 (2.4) |
| Uppsala, Sweden | 10.6 (1.9) | 8.8 (1.9) | 19.1 (2.5) | 14.7 (2.4) | 29.1 (2.8) | 21.6 (2.8) |
| Lexington, USA | 13.2 (2.5) | 15.2 (2.5) | 19.6 (3.0) | 19.3 (2.8) | 25.2 (3.3) | 21 (2.9) |
| Krakow, Poland | 13.6 (2.1) | 11.6 (2.0) | 27.0 (2.5) | 16.2 (2.3) | 32.6 (2.8) | 20.3 (2.6) |
| Salzburg, Austria | 13.6 (1.4) | 20.9 (1.9) | 27.2 (1.8) | 26.0 (2.1) | 37.5 (2.0) | 37.1 (2.3) |
| Vancouver, Canada | 14.2 (2.0) | 12.2 (1.6) | 21.3 (2.4) | 16.3 (1.8) | 28.2 (2.6) | 22.9 (2.0) |
| Bergen, Norway | 14.5 (2.0) | 10.2 (1.8) | 23.2 (2.4) | 15.3 (2.0) | 33.4 (2.7) | 27.3 (2.6) |
| Phililppines, urban | 15.7 (2.5) | 4.3 (0.8) | 20.5 (2.0) | 8.2 (1.1) | 27.2 (2.2) | 9.9 (1.6) |
| Phililppines, rural | 17.4 (1.9) | 13.9 (2.4) | 26.5 (2.7) | 15.4 (2.5) | 29.3 (2.1) | 21.8 (2.1) |
| London, UK | 19.6 (3.2) | 16.2 (2.9) | 29.5 (3.5) | 19.3 (3.1) | 38.4 (3.5) | 26.7 (3.4) |
| Adana, Turkey | 20.0 (2.1) | 8.9 (1.8) | 28.8 (2.0) | 10.4 (2.0) | 40.8 (2.5) | 17.8 (2.4) |
| Cape Town, S.Africa | 21.8 (2.5) | 16.3 (1.8) | 27.3 (2.7) | 19.6 (2.0) | 32.9 (2.9) | 22.3 (22.3) |
Analytic Methods
Given the limited number of centres and the need to derive standard estimates from the published literature, we have computed a simple ratio to adjust other studies’ prevalence estimates to a definition based on post-bronchodilator spirometry using the LLN reference values derived from the NHANES survey. (8)
If we have a prevalence based on an alternative definition (P’) and we wish to calculate the equivalent prevalence using our “gold standard” definition (P) we first use the BOLD data to derive a conversion ratio P/P’. In any new survey (S) that has estimated the prevalence using the alternative definition (P’S), we then estimate the “gold standard” prevalence in that population (PS) from:
This can also be calculated for specific age (i) and sex (j) groups: PSij = P’Sij*Pij/P’ij.
We have estimated the conversion ratios using the BOLD data from two of the most commonly found alternative estimates in the literature, the prevalence of a pre-bronchodilator FEV1/FVC of less than 70% and also to a post-bronchodilator FEV1/FVC of less than 70% and converted these to the “gold standard” used in BOLD of a post-bronchodilator FEV1/FVC less than the lower limit of normal (conversion ratios for further outcomes are available in the web annex).
To validate the performance of these ratios in independent datasets, this comparison was repeated using the ratios calculated using the initial 16 centres in two further available datasets: six more recently completed BOLD surveys and five Latin American centres from the PLATINO study. (7) To provide a conservative estimate of performance we used a sex-specific, all-ages (40+ years) ratio. We have plotted the differences in derived estimates from the directly measured estimates of the prevalence of a FEV1/FVC < LLN in a Bland-Altman plot. (9)
Results
Table 1 gives the estimated prevalence of CAO using three different definitions (post-bronchodilator FEV1/FVC<LLN (“gold standard”), post-bronchodilator FEV1/FVC<70% and pre-bronchodilator FEV1/FVC<70%). Compared with the ratio < LLN definition the GOLD definition (post-bronchodilator ratio < 70%) gives higher prevalences and the pre-bronchodilator ratio < 70% definition higher prevalences still.
Table 2 gives the age-sex specific and sex-specific conversion ratios for participants over the age of 40 years derived from the initial 16 BOLD sites. Compared to the reference definition, the use of a fixed ratio generally increases the estimated prevalence of CAO, more so in older participants, whilst not using a bronchodilator tends to increase the estimated prevalence of disease even more.
Table 2.
Age-sex specific and sex-specific conversion ratios to estimate the prevalence of reference Chronic Obstructive Airways (COA) disease (post-bronchodilator FEV1/FVC < Lower Limit of Normal) from two commonly presented COA prevalence estimates.
| Post-bronchodilator FEV1/FVC<LLN | Post-bronchodilator FEV1/FVC<70% | Pre-bronchodilator FEV1/FVC<70% | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Age group | Male | Female | Male | Female | Male | Female |
|
| ||||||
| 40-49 years | 1 | 1 | 0.83 | 1.57 | 0.54 | 0.87 |
| 50-59 years | 1 | 1 | 0.71 | 0.88 | 0.57 | 0.54 |
| 60-69 years | 1 | 1 | 0.45 | 0.71 | 0.35 | 0.39 |
| 70+ years | 1 | 1 | 0.50 | 0.54 | 0.36 | 0.34 |
|
| ||||||
| 40+ years | 1 | 1 | 0.59 | 0.80 | 0.44 | 0.47 |
Note.
For example, to obtain the reference prevalence(post-bronchodilator FEV1/FVC<LLN) in males aged 50-59 given the prevalence in that age-sex group of COA using the definition of pre-bronchodilator FEV1/FVC<70%, multiply the given prevalence by 0.57.
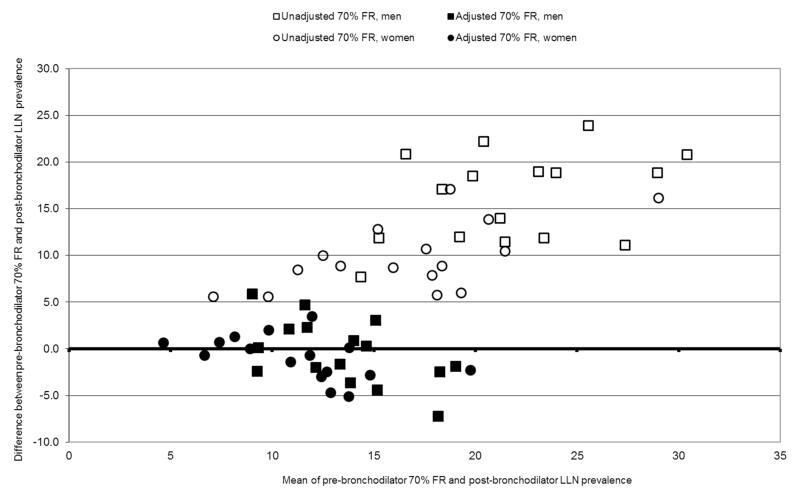
The estimates of obstruction given by a pre-bronchodilator FEV1/FVC < 70% fixed cut off point and the adjusted estimate (derived by applying the conversion ratios to the same data) were each compared with the reference estimate based on a post-bronchodilator FEV1/FVC <LLN. Figure 1 shows this as a Bland-Altman plot with the differences between these estimates and the “gold standard” on the y-axis and the means of these estimates and the “gold standard” on the x-axis. The unadjusted pre-bronchodilator ratio <70% (open squares (men) and open circles (women)) is a poor estimate of the post-bronchodilator ratio < lower limit of normal, giving values that increase with prevalence (that is, show bias) and are consistently between 5% and 25% points higher than the directly measured reference value. The adjusted values, estimated using the conversion factor (closed squares (men); closed circles (women)) are by definition, on average, the same as the true values, but appear independent of prevalence rate and are almost all confined between +/− 5% points of the measured values.
Figure 1.
Bland-Altman plot comparing unadjusted and adjusted pre-bronchodilator 70% fixed ratio prevalence estimates with post-bronchodilator LLN prevalence in men and women, in the initial 16 BOLD centres.
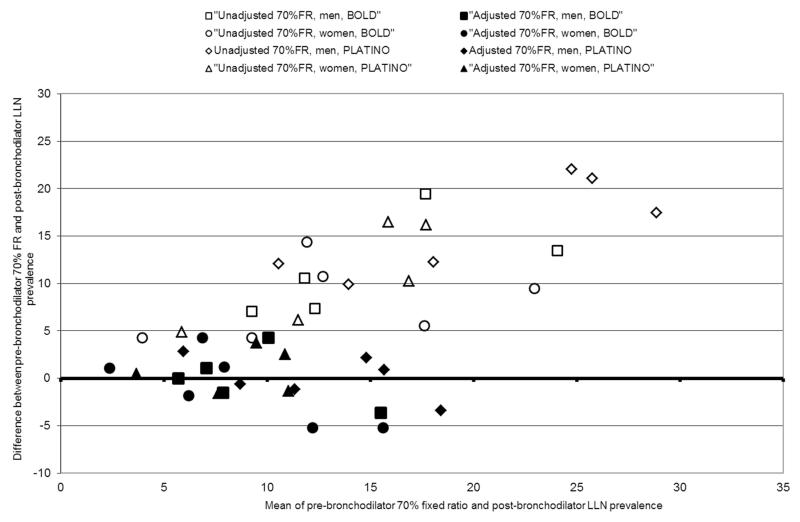
To verify that the ratios derived from BOLD were effective in independent datasets this comparison was repeated on two further available datasets: six later BOLD surveys and five Latin American centres in PLATINO, an international study using a similar protocol to BOLD. Table 3 gives the directly measured prevalence of COPD in these eleven test sites using the same definitions as before. A similar pattern is seen again in which the post-bronchodilator ratio<70% gives rise to higher prevalences than seen with our “gold standard” definition and the pre-bronchodilator ratio<70% gives higher prevalences still. Figure 2 is the Bland-Altman plot comparing the post-bronchodilator ratio < LLN with the pre-bronchodilator ratio < 70% cut-off before and after adjustment using the conversion ratios derived from the analysis of the initial 16 BOLD sites and compared with the “gold standard” of directly measured post-bronchodilator ratio < LLN. The unadjusted values are all between 4 and 23% higher than the directly measured result, whereas the adjusted results all fall within 5% of the directly observed result and are more evenly distributed around it.
Table 3.
Directly observed prevalence of COA in 5 PLATINO and 6 more recent BOLD sites using different definitions of COA.
| Test site | Post-bronchodilator FEV1/FVC<LLN | Post-bronchodilator FEV1/FVC<70% | Pre-bronchodilator FEV1/FVC<70% | |||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
|
| ||||||
| Mexico City, Mexico (P) | 4.5 (0.9) | 3.4 (0.8) | 11.1 (1.7) | 5.3 (1.0) | 16.6 (2.0) | 8.3 (1.2) |
| Caracas, Venezuela (P) | 9.0 (1.6) | 8.4 (1.1) | 15.6 (1.6) | 9.9 (1.0) | 18.9 (1.7) | 14.6 (1.1) |
| Sao Paulo, Brazil (P) | 11.9 (1.5) | 11.7 (1.4) | 17.5 (1.7) | 14.2 (1.5) | 24.2 (1.9) | 22.0 (2.1) |
| Santiago, Chile (P) | 13.7 (1.4) | 7.6 (1.1) | 23.2 (1.8) | 11.8 (1.3) | 35.8 (2.3) | 24.1 (1.7) |
| Montevideo, Uruguay (P) | 15.2 (1.9) | 9.6 (1.3) | 26.9 (2.2) | 14.5 (1.5) | 36.3 (2.3) | 25.8 (1.8) |
| Maastricht, Netherlands (B) | 20.1 (2.5) | 18.2 (2.9) | 29.1 (2.9) | 20.0 (3.1) | 37.6 (3.0) | 27.8 (3.2) |
| Mumbai, India (B) | 5.7 (1.4) | 7.3 (2.3) | 9.4 (1.8) | 7.1 (2.4) | 12.8 (2.1) | 18.1 (5.2) |
| Pune, India (B) | 6.5 (1.2) | 7.1 (1.6) | 11.2 (1.5) | 7.1 (1.6) | 17.1 (1.8) | 11.4 (1.9) |
| Sousse, Tunisia (B) | 8.6 (2.1) | 1.8 (0.7) | 13.6 (2.7) | 1.9 (0.7) | 16.0 (2.9) | 6.1 (1.3) |
| Srinagar, India (B) | 17.4 (3.9) | 14.8 (2.2) | 23.4 (4.7) | 14.8 (2.1) | 30.8 (5.6) | 20.4 (3.2) |
| Tartu, Estonia (B) | 7.9 (1.5) | 4.7 (1.2) | 16.5 (2.1) | 10.4 (1.8) | 27.4 (2.6) | 19.1 (2.3) |
Figure 2.
Bland-Altman plot comparing unadjusted and adjusted pre-bronchodilator 70% fixed ratio prevalence estimates with post-bronchodilator LLN prevalence in men and women, PLATINO and further 6 BOLD centres.
Discussion
The wide variety of outcome measures currently used to estimate the prevalence of COPD in published studies makes comparisons across global regions and over time uninformative (when using different definitions) or limited (when using only one). Using the largest international study of COPD prevalence, we have derived conversion ratios which enable such comparisons to be made using the prevalence of post-bronchodilator FEV1/FVC<LLN as the reference method. The resulting adjusted prevalence estimates, when applied to both BOLD and other independent study samples, do not vary systematically by prevalence and in each case provide prevalence estimates very much closer to the outcome of choice. The conversion ratios are presented here for four decennial age categories in the over 40s as well as for all-ages over 40 years. The accuracy of the adjusted estimates in men and women of all-ages over 40 years are also illustrated as a conservative estimate of the efficacy of the ratio, but tests were made in age-sex specific samples and show similar results.
There is good agreement that COPD should be defined by the ratio of post-bronchodilator FEV1/ FVC, but less agreement on what criterion should be used. The GOLD guidelines recommend that the fixed cut off of 70% should be used at all age groups. This has the advantage of simplicity, though this seems a minor consideration now that all spirometers include powerful microchips and can easily report the lower limits of normal.
The disadvantage in using the fixed cut-off is that ventilatory function declines with age. Even those few who argue that this decline, though universal, should still be treated as pathological, generally adjust their analyses for age. This is possible when analysing individual data, but not when analysing grouped data (such as prevalence). Here the great diversity in the age structures of populations makes any prevalence based on the fixed ratio very difficult to interpret.
The usual solution to this problem is to select only “GOLD stage II” disease. (10) This is defined as a FEV1/FVC ratio < 70% and a FEV1 < 80% of the predicted FEV1 for age, sex and height. This has the effect of at least partially adjusting the measurement for age. However it makes the initial problem even worse. First, because the distribution of the predicted value of FEV1 decreases with age, the 80% deficit in predicted FEV1 also slightly underestimates the lower limit of normal. Second, because the FEV1 is influenced not just by the obstruction of the airway but also by the size of the lungs, its use confuses restriction, which also increases with age, with obstruction. GOLD Stage II is a hybrid measure of both low FVC and low FEV1/FVC ratio.(11)
BOLD and PLATINO use a 200 μg dose of salbutamol administered via a spacer to assess post-bronchodilator ventilatory function, which is lower than the 400mg dose recommended by GOLD. As these are surveys in normal subjects not used to taking inhaled β-agonists it was felt that the side-effects that are common at 400μg would not be acceptable and it was noted that the effective maximum bronchodilation in normal subjects occurs somewhere between 100 and 300 μgs.(12)
The GOLD guidelines have been successful in reducing the variability of reporting but there is still a very wide variation in practice. In a recent unpublished review we found 10 different variations of indices used to diagnose COPD based on the FEV1/FVC ratio including
FEV1/FVC<60%, FEV1/FVC<80%, FEV1/FVC<88 % predicted for men; < 89% predicted for women and 70%≤FEV1/FVC<80%, with yet more definitions not based on the FEV1/FVC ratio. It would be useful to see this harmonisation increase, but even better if the standard moved away from the fixed cut off. In the meantime there will be a need for methods to standardise the very different estimates from the published sources. This paper goes some way to filling this gap.
Supplementary Material
Table 2a. Age-sex specific and sex-specific conversion ratios to estimate the prevalence of reference COA (post-bronchodilator FEV1/FVC < Lower Limit of Normal) from further commonly presented COA prevalence estimates and their respective modified GOLD II-IV estimates.
ACKNOWLEDGMENTS
RG was funded by BUPA.
The BOLD Study is currently funded by a grant from the Wellcome Trust (085790/Z/08/Z).
The initial BOLD programme was funded in part by unrestricted educational grants to the Operations Center in Portland, Oregon, from ALTANA, Aventis, AstraZeneca, Boehringer-Ingelheim, Chiesi, GlaxoSmithKline, Merck, Novartis, Pfizer, Schering-Plough, Sepracor, and the University of Kentucky.
Additional local support for BOLD sites
Additional local support for BOLD sites was provided by: Boehringer Ingelheim China. (GuangZhou, China ); Turkish Thoracic Society, Boehringer-Ingelheim, and Pfizer (Adana, Turkey ); Altana, Astra-Zeneca, Boehringer-Ingelheim, GlaxoSmithKline, Merck Sharpe & Dohme, Novartis, Salzburger Gebietskrankenkasse and Salzburg Local Government (Salzburg, Austria ); Research for International Tobacco Control, the International Development Research Centre, the South African Medical Research Council, the South African Thoracic Society GlaxoSmithKline Pulmonary Research Fellowship, and the University of Cape Town Lung Institute (Cape Town, South Africa ); and Landspítali-University Hospital-Scientific Fund, GlaxoSmithKline Iceland, and AstraZeneca Iceland (Reykjavik, Iceland ); GlaxoSmithKline Pharmaceuticals, Polpharma, Ivax Pharma Poland, AstraZeneca Pharma Poland, ZF Altana Pharma, Pliva Kraków, Adamed, Novartis Poland, Linde Gaz Polska, Lek Polska, Tarchomińskie Zakłady Farmaceutyczne Polfa, Starostwo Proszowice, Skanska, Zasada, Agencja Mienia Wojskowego w Krakowie, Telekomunikacja Polska, Biernacki, Biogran, Amplus Bucki, Skrzydlewski, Sotwin, and Agroplon (Krakow, Poland); Boehringer-Ingelheim, and Pfizer Germany (Hannover, Germany ); the Norwegian Ministry of Health’s Foundation for Clinical Research, and Haukeland University Hospital’s Medical Research Foundation for Thoracic Medicine (Bergen, Norway); AstraZeneca, Boehringer-Ingelheim, Pfizer, and GlaxoSmithKline (Vancouver, Canada); Marty Driesler Cancer Project (Lexington, Kentucky, USA); Altana, Boehringer Ingelheim (Phil), GlaxoSmithKline, Pfizer, Philippine College of Chest Physicians, Philippine College of Physicians, and United Laboratories (Phil) (Manila, Philippines); Air Liquide Healthcare P/L, AstraZeneca P/L, Boehringer Ingelheim P/L, GlaxoSmithKline Australia P/L, Pfizer Australia P/L (Sydney, Australia), Department of Health Policy Research Programme, Clement Clarke International (London, United Kingdom); Boehringer Ingelheim and Pfizer (Lisbon, Portugal), Swedish Heart and Lung Foundation, The Swedish Association against Heart and Lung Diseases, Glaxo Smith Kline (Uppsala, Sweden); GlaxoSmithKline, Astra Zeneca, Eesti Teadusfond (Estonian Science Foundation) (Tartu, Estonia); AstraZeneca, CIRO HORN (Maastricht, The Netherlands); Sher-i-Kashmir Institute of Medical Sciences, Srinagar, J&K (Srinagar, India); Foundation for Environmental Medicine, Kasturba Hospital, Volkart Foundation (Mumbai, India); Boehringer Ingelheim (Sousse, Tunisia); Philippines College of Physicians, Philippines College of Chest Physicians, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline , Orient Euro Pharma, Otsuka Pharma , United laboratories Phillipines (Nampicuan&Talugtug, Philippines). The sponsors of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
The BOLD Collaboration
A Sonia Buist, William Vollmer, Suzanne Gillespie, MaryAnn McBurnie (BOLD Co-ordinating Centre, Kaiser Permanente Portland, USA), Peter GJ Burney, Anamika Jithoo, Louisa Gnatiuc, Bernet Kato, Sonia Coton, Hadia Azhar (BOLD Co-ordinating Centre, Imperial College London, UK), NanShan Zhong (PI), Shengming Liu, Jiachun Lu, Pixin Ran, Dali Wang, Jingping Zheng, Yumin Zhou (Guangzhou Institute of Respiratory Diseases, Guangzhou Medical College, Guangzhou, China); Ali Kocabaş (PI), Attila Hancioglu, Ismail Hanta, Sedat Kuleci, Ahmet Sinan Turkyilmaz, Sema Umut, Turgay Unalan (Cukurova University School of Medicine, Department of Chest Diseases, Adana, Turkey); Michael Studnicka (PI), Torkil Dawes, Bernd Lamprecht, Lea Schirhofer (Paracelsus Medical University, Department of Pulmonary Medicine, Salzburg Austria); Eric Bateman (PI), Anamika Jithoo (PI), Desiree Adams, Edward Barnes, Jasper Freeman, Anton Hayes, Sipho Hlengwa, Christine Johannisen, Mariana Koopman, Innocentia Louw, Ina Ludick, Alta Olckers, Johanna Ryck, Janita Storbeck, (University of Cape Town Lung Institute, Cape Town, South Africa); Thorarinn Gislason (PI), Bryndis Benedikdtsdottir, Kristin Jörundsdottir, Lovisa Gudmundsdottir, Sigrun Gudmundsdottir, Gunnar Gundmundsson, (Landspitali University Hospital, Dept. of Allergy, Respiratory Medicine and Sleep, Reykjavik, Iceland); Ewa Nizankowska-Mogilnicka (PI) , Jakub Frey, Rafal Harat, Filip Mejza, Pawel Nastalek, Andrzej Pajak, Wojciech Skucha, Andrzej Szczeklik,Magda Twardowska, (Division of Pulmonary Diseases, Department of Medicine, Jagiellonian University School of Medicine, Cracow, Poland); Tobias Welte (PI), Isabelle Bodemann, Henning Geldmacher, Alexandra Schweda-Linow (Hannover Medical School, Hannover, Germany); Amund Gulsvik (PI), Tina Endresen, Lene Svendsen (Department of Thoracic Medicine, Institute of Medicine, University of Bergen, Bergen, Norway); Wan C. Tan (PI), Wen Wang (iCapture Center for Cardiovascular and Pulmonary Research, University of British Columbia, Vancouver, BC, Canada); David M. Mannino (PI), John Cain, Rebecca Copeland, Dana Hazen, Jennifer Methvin, (University of Kentucky, Lexington, Kentucky, USA); Renato B. Dantes (PI), Lourdes Amarillo, Lakan U. Berratio, Lenora C. Fernandez, Norberto A. Francisco, Gerard S. Garcia, Teresita S. de Guia, Luisito F. Idolor, Sullian S. Naval, Thessa Reyes, Camilo C. Roa, Jr., Ma. Flordeliza Sanchez, Leander P. Simpao (Philippine College of Chest Physicians, Manila, Philippines); Christine Jenkins (PI), Guy Marks (PI), Tessa Bird, Paola Espinel, Kate Hardaker, Brett Toelle (Woolcock Institute of Medical Research, Sydney, Australia), Peter GJ Burney (PI), Caron Amor, James Potts, Michael Tumilty, Fiona McLean (National Heart and Lung Institute, Imperial College, London), E.F.M. Wouters, G.J. Wesseling (Maastricht University Medical Center, Maastricht, the Netherlands), Cristina Bárbara (PI), Fátima Rodrigues, Hermínia Dias, João Cardoso, João Almeida, Maria João Matos, Paula Simão, Moutinho Santos, Reis Ferreira (The Portuguese Society of Pneumology, Lisbon, Portugal), Christer Janson (PI), Inga Sif Olafsdottir, Katarina Nisser, Ulrike Spetz-Nyström, Gunilla Hägg and Gun-Marie Lund (Department of Medical Sciences: Respiratory Medicine & Allergology, Uppsala University, Sweden), Rain Jõgi (PI), Hendrik Laja, Katrin Ulst, Vappu Zobel, Toomas-Julius Lill (Lung Clinic, Tartu University Hospital), Parvaiz A Koul (PI), Sajjad Malik, Nissar A Hakim, Umar Hafiz Khan (Sher-i-Kashmir Institute of Medical Sciences, Srinagar, J&K, India); Rohini Chowgule (PI) Vasant Shetye, Jonelle Raphael, Rosel Almeda, Mahesh Tawde, Rafiq Tadvi, Sunil Katkar, Milind Kadam, Rupesh Dhanawade, Umesh Ghurup (Indian Institute of Environmental Medicine, Mumbai, India); Imed Harrabi (PI), Myriam Denguezli, Zouhair Tabka, Hager Daldoul, Zaki Boukheroufa, Firas Chouikha, Wahbi Belhaj Khalifa (Faculté de Médecine, Sousse, Tunisia); Luisito F. Idolor (PI), Teresita S. de Guia, Norberto A. Francisco, Camilo C. Roa, Fernando G. Ayuyao, Cecil Z.Tady, Daniel T. Tan, Sylvia Banal-Yang, Vincent M. Balanag, Jr., Maria Teresita N. Reyes, Renato. B. Dantes (Lung Centre of the Philippines, Philippine General Hospital, Nampicuan&Talugtug, Philippines); Sundeep Salvi (PI), Sundeep Salvi (PI), Siddhi Hirve, Bill Brashier, Jyoti Londhe , Sapna Madas, Somnath Sambhudas, Bharat Chaidhary, Meera Tambe, Savita Pingale, Arati Umap, Archana Umap, Nitin Shelar, Sampada Devchakke, Sharda Chaudhary, Suvarna Bondre, Savita Walke, Ashleshsa Gawhane, Anil Sapkal, Rupali Argade, Vijay Gaikwad (Vadu HDSS, KEM Hospital Research Centre Pune, Chest Research Foundation (CRF), Pune India).
References
- (1).Murray CJL, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- (2).Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. [Review] [88 refs] Eur Respir J. 2006;28(3):523–532. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- (3).Global Initiative for Chronic Obstructive Lung Disease [Accessed Nov 2012];Global Strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. 20011; Available at: www.goldcopd.org.
- (4).Swanney MP, Ruppel G, Enright PL, Pedersen OF, Crapo RO, Miller MR, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008;63(12):1046–51. doi: 10.1136/thx.2008.098483. [DOI] [PubMed] [Google Scholar]
- (5).Buist AS, Vollmer WM, Sullivan SD, Weiss KB, Lee TA, Menezes AM, et al. The Burden of Obstructive Lung Disease Initiative (BOLD): rationale and design. COPD. 2005 Jun;2(2):277–283. [PubMed] [Google Scholar]
- (6).Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741–50. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- (7).Menezes AM, Perez-Padilla R, Jardim JR, Muino A, Lopez MV, Valdivia G, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study.[see comment] Lancet. 2005 Nov 26;366(9500):1875–1881. doi: 10.1016/S0140-6736(05)67632-5. [DOI] [PubMed] [Google Scholar]
- (8).Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- (9).Bland JM, Altman DG. Statistical Methods for Assessing Agreement betweenTwo Methods of Clinical Measurement. Lancet. 1986;(2):307–310. [PubMed] [Google Scholar]
- (10).Vollmer WM, Gíslason T, Burney P, Enright PL, Gulsvik A, Kocabas A, et al. Comparison of spirometry criteria for the diagnosis of COPD: results from the BOLD study. Eur Respir J. 2009;34(3):588–97. doi: 10.1183/09031936.00164608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Burney PGJ, Hooper R. Forced vital capacity, airway obstruction and survival in a general population sample from the USA. Thorax. 2011;66(1):49–54. doi: 10.1136/thx.2010.147041. [DOI] [PubMed] [Google Scholar]
- (12).Bergendal A, Johansson Å , Bake B, Lötvall J, Skoogh B-E, Löfdahl C-G. Airway Effects of Salmeterol in Healthy Individuals. Pulmonary Pharmacology. 1995;8:283–8. doi: 10.1006/pulp.1995.1038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 2a. Age-sex specific and sex-specific conversion ratios to estimate the prevalence of reference COA (post-bronchodilator FEV1/FVC < Lower Limit of Normal) from further commonly presented COA prevalence estimates and their respective modified GOLD II-IV estimates.