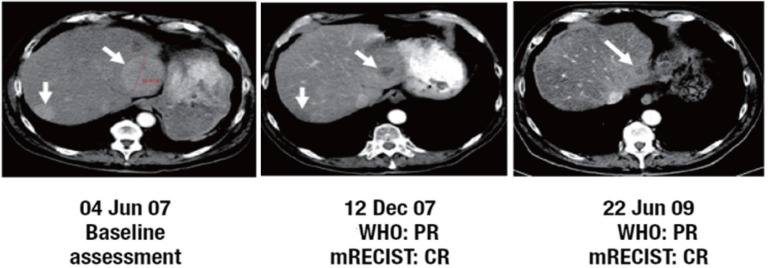
Fig. 1.
Example of a patient treated with first-line brivanib alaninate 800 mg once daily (cohort A) who was initially assessed as having PR by WHO criteria. On scan reassessment by mRECIST, this patient was classified as having CR. Adapted with permission from Park JW, Finn RS, Kim JS, et al. Phase II, open-label study of brivanib as first-line therapy in patients with advanced hepatocellular carcinoma. As described by Park JW, et al. [15].