Fig. 8.
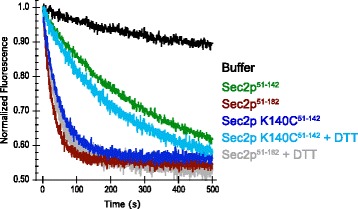
Disulfide bond introduction between Sec2p monomers increases protein activity. MantGDP dissociation from Sec4p was measured by reduction in fluorescence intensity after mixing excess of unlabeled GDP (300 μM) plus: buffer or 250nM of different Sec2p constructs. The mutant K140C increases the rather low dissociation rate Sec2p51–142. When DTT (50 mM) is added to the buffer solution the dissociation level drops close to the wild type level. The addition of DTT to the truncation Sec2p51–182 is not deleterious to the protein activity. All assays were performed with 1 μM Sec4p19–187 preloaded with mantGDP
