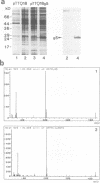
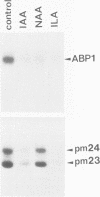
Abstract
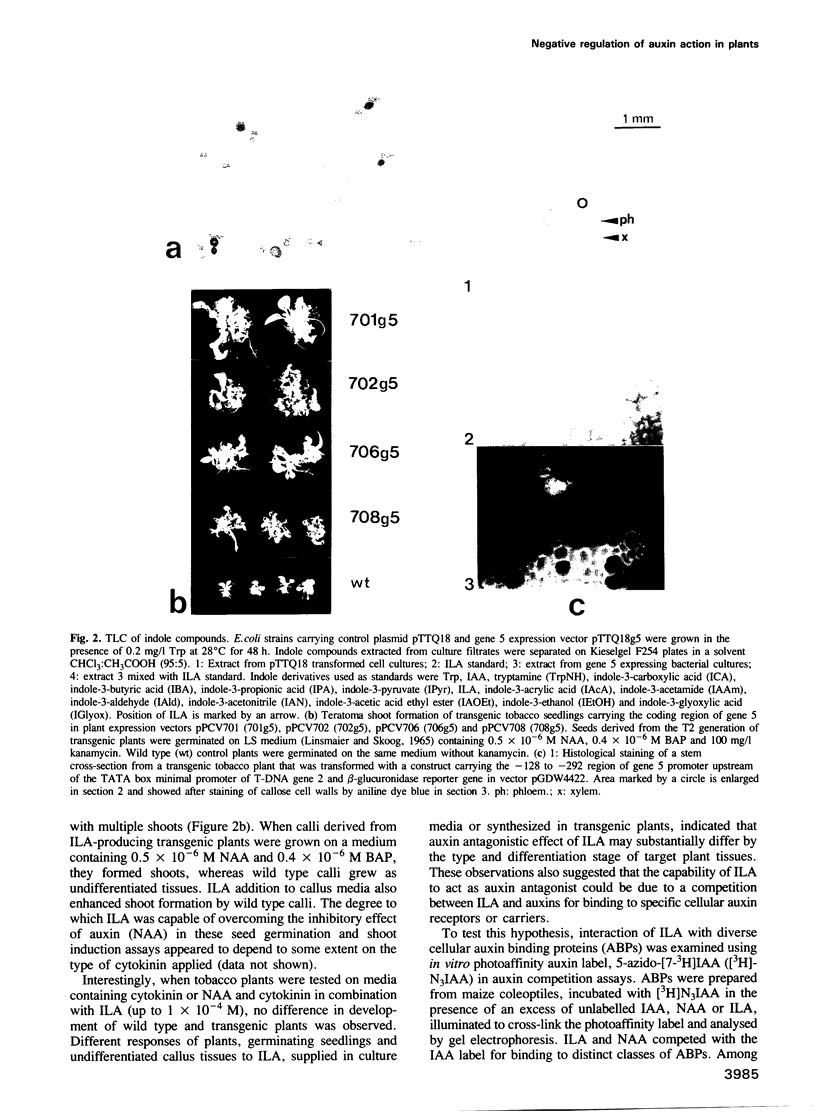
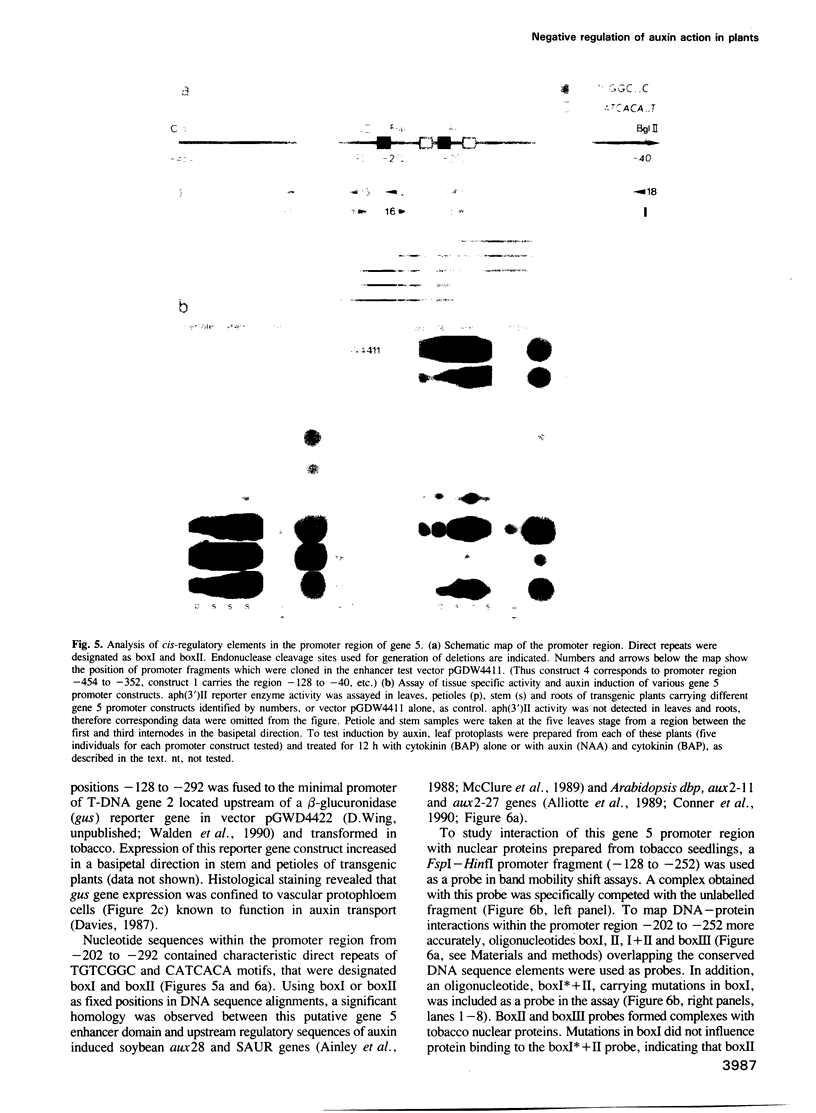
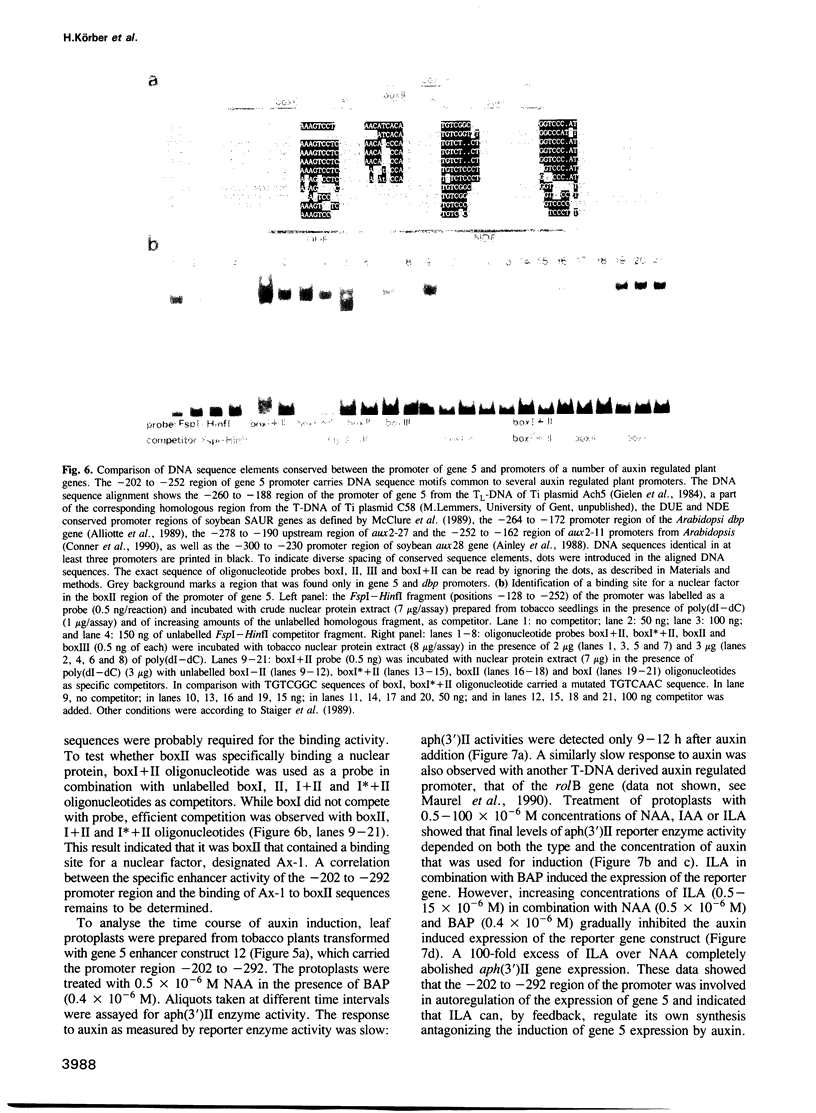
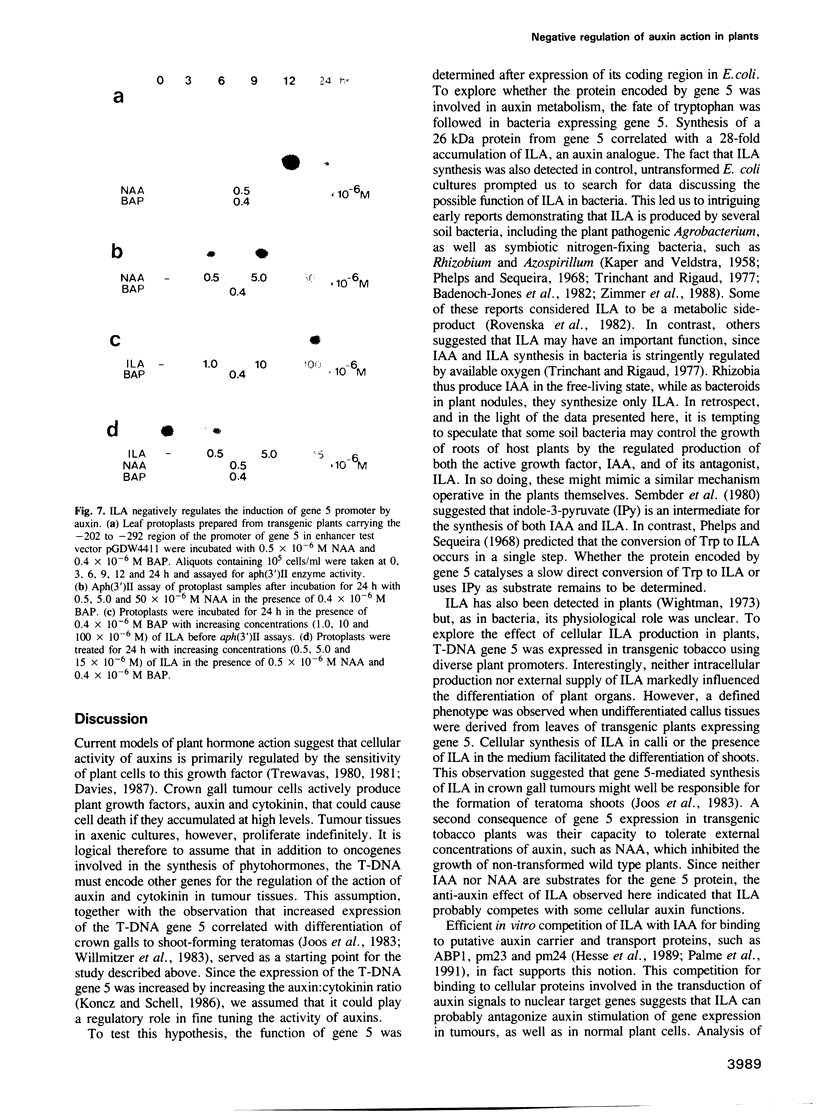
Oncogenes carried by the transferred DNA (T-DNA) of Agrobacterium Ti plasmids encode the synthesis of plant growth factors, auxin and cytokinin, and induce tumour development in plants. Other T-DNA genes regulate the tumorous growth in ways that are not yet understood. To determine the function of T-DNA gene 5, its coding region was expressed in Escherichia coli. Synthesis of the gene 5 encoded protein (26 kDa) correlated with a 28-fold increase in conversion of tryptophan to indole-3-lactate (ILA), an auxin analogue. Expression of chimeric gene 5 constructs in transgenic tobacco resulted in overproduction of ILA that enhanced shoot formation in undifferentiated tissues and increased the tolerance of germinating seedlings to the inhibitory effect of externally supplied auxin. Promoter analysis of gene 5 in plants revealed that its expression was inducible by auxin and confined to the vascular phloem cells. cis-regulatory elements required for auxin regulation and phloem specific expression of gene 5 were mapped to a 90 bp promoter region that carried DNA sequence motifs common to several auxin induced plant promoters, as well as a binding site for a nuclear factor, Ax-1. ILA was found to inhibit the auxin induction of the gene 5 promoter and to compete with indole-3-acetic acid (IAA) for in vitro binding to purified cellular auxin binding proteins. It is suggested therefore that ILA autoregulates its own synthesis and thereby modulates a number of auxin responses in plants.
Full text
PDF
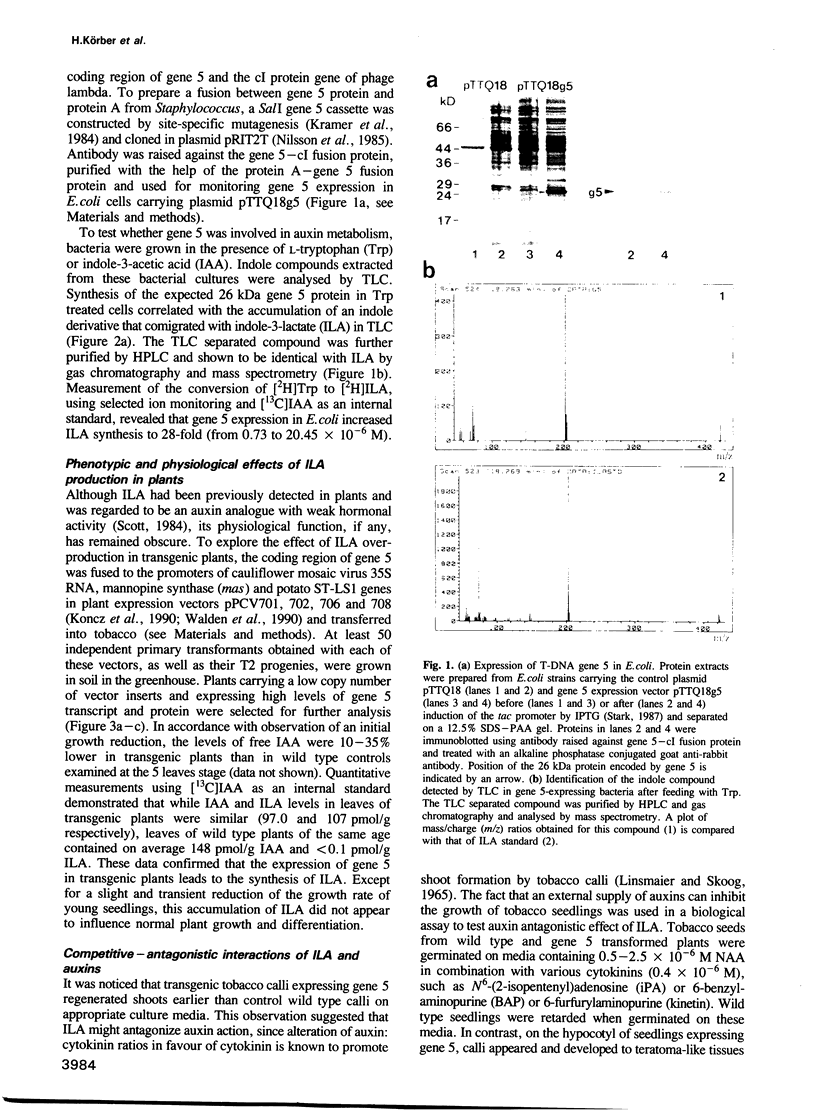



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ainley W. M., Walker J. C., Nagao R. T., Key J. L. Sequence and characterization of two auxin-regulated genes from soybean. J Biol Chem. 1988 Aug 5;263(22):10658–10666. [PubMed] [Google Scholar]
- Akiyoshi D. E., Klee H., Amasino R. M., Nester E. W., Gordon M. P. T-DNA of Agrobacterium tumefaciens encodes an enzyme of cytokinin biosynthesis. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5994–5998. doi: 10.1073/pnas.81.19.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alliotte T., Tiré C., Engler G., Peleman J., Caplan A., Van Montagu M., Inzé D. An Auxin-Regulated Gene of Arabidopsis thaliana Encodes a DNA-Binding Protein. Plant Physiol. 1989 Mar;89(3):743–752. doi: 10.1104/pp.89.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner T. W., Goekjian V. H., LaFayette P. R., Key J. L. Structure and expression of two auxin-inducible genes from Arabidopsis. Plant Mol Biol. 1990 Oct;15(4):623–632. doi: 10.1007/BF00017836. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielen J., De Beuckeleer M., Seurinck J., Deboeck F., De Greve H., Lemmers M., Van Montagu M., Schell J. The complete nucleotide sequence of the TL-DNA of the Agrobacterium tumefaciens plasmid pTiAch5. EMBO J. 1984 Apr;3(4):835–846. doi: 10.1002/j.1460-2075.1984.tb01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse T., Feldwisch J., Balshüsemann D., Bauw G., Puype M., Vandekerckhove J., Löbler M., Klämbt D., Schell J., Palme K. Molecular cloning and structural analysis of a gene from Zea mays (L.) coding for a putative receptor for the plant hormone auxin. EMBO J. 1989 Sep;8(9):2453–2461. doi: 10.1002/j.1460-2075.1989.tb08380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John M., Schmidt J., Wieneke U., Kondorosi E., Kondorosi A., Schell J. Expression of the nodulation gene nod C of Rhizobium meliloti in Escherichia coli: role of the nod C gene product in nodulation. EMBO J. 1985 Oct;4(10):2425–2430. doi: 10.1002/j.1460-2075.1985.tb03951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joos H., Inzé D., Caplan A., Sormann M., Van Montagu M., Schell J. Genetic analysis of T-DNA transcripts in nopaline crown galls. Cell. 1983 Apr;32(4):1057–1067. doi: 10.1016/0092-8674(83)90290-8. [DOI] [PubMed] [Google Scholar]
- KAPER J. M., VELDSTRA H. On the metabolism of tryptophan by Agrobacterium tumefaciens. Biochim Biophys Acta. 1958 Nov;30(2):401–420. doi: 10.1016/0006-3002(58)90065-9. [DOI] [PubMed] [Google Scholar]
- Koncz C., Martini N., Mayerhofer R., Koncz-Kalman Z., Körber H., Redei G. P., Schell J. High-frequency T-DNA-mediated gene tagging in plants. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8467–8471. doi: 10.1073/pnas.86.21.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncz C., Mayerhofer R., Koncz-Kalman Z., Nawrath C., Reiss B., Redei G. P., Schell J. Isolation of a gene encoding a novel chloroplast protein by T-DNA tagging in Arabidopsis thaliana. EMBO J. 1990 May;9(5):1337–1346. doi: 10.1002/j.1460-2075.1990.tb08248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer W., Drutsa V., Jansen H. W., Kramer B., Pflugfelder M., Fritz H. J. The gapped duplex DNA approach to oligonucleotide-directed mutation construction. Nucleic Acids Res. 1984 Dec 21;12(24):9441–9456. doi: 10.1093/nar/12.24.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C., Brevet J., Barbier-Brygoo H., Guern J., Tempé J. Auxin regulates the promoter of the root-inducing rolB gene of Agrobacterium rhizogenes in transgenic tobacco. Mol Gen Genet. 1990 Aug;223(1):58–64. doi: 10.1007/BF00315797. [DOI] [PubMed] [Google Scholar]
- Mayerhofer R., Koncz-Kalman Z., Nawrath C., Bakkeren G., Crameri A., Angelis K., Redei G. P., Schell J., Hohn B., Koncz C. T-DNA integration: a mode of illegitimate recombination in plants. EMBO J. 1991 Mar;10(3):697–704. doi: 10.1002/j.1460-2075.1991.tb07999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure B. A., Hagen G., Brown C. S., Gee M. A., Guilfoyle T. J. Transcription, organization, and sequence of an auxin-regulated gene cluster in soybean. Plant Cell. 1989 Feb;1(2):229–239. doi: 10.1105/tpc.1.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson B., Abrahmsén L., Uhlén M. Immobilization and purification of enzymes with staphylococcal protein A gene fusion vectors. EMBO J. 1985 Apr;4(4):1075–1080. doi: 10.1002/j.1460-2075.1985.tb03741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palme K., Hesse T., Moore I., Campos N., Feldwisch J., Garbers C., Hesse F., Schell J. Hormonal modulation of plant growth: the role of auxin perception. Mech Dev. 1991 Feb;33(2):97–106. doi: 10.1016/0925-4773(91)90076-i. [DOI] [PubMed] [Google Scholar]
- Reiss B., Sprengel R., Will H., Schaller H. A new sensitive method for qualitative and quantitative assay of neomycin phosphotransferase in crude cell extracts. Gene. 1984 Oct;30(1-3):211–217. doi: 10.1016/0378-1119(84)90122-7. [DOI] [PubMed] [Google Scholar]
- Schröder G., Waffenschmidt S., Weiler E. W., Schröder J. The T-region of Ti plasmids codes for an enzyme synthesizing indole-3-acetic acid. Eur J Biochem. 1984 Jan 16;138(2):387–391. doi: 10.1111/j.1432-1033.1984.tb07927.x. [DOI] [PubMed] [Google Scholar]
- Spanier K., Schell J., Schreier P. H. A functional analysis of T-DNA gene 6b: the fine tuning of cytokinin effects on shoot development. Mol Gen Genet. 1989 Oct;219(1-2):209–216. doi: 10.1007/BF00261179. [DOI] [PubMed] [Google Scholar]
- Spena A., Schmülling T., Koncz C., Schell J. S. Independent and synergistic activity of rol A, B and C loci in stimulating abnormal growth in plants. EMBO J. 1987 Dec 20;6(13):3891–3899. doi: 10.1002/j.1460-2075.1987.tb02729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark M. J. Multicopy expression vectors carrying the lac repressor gene for regulated high-level expression of genes in Escherichia coli. Gene. 1987;51(2-3):255–267. doi: 10.1016/0378-1119(87)90314-3. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmitzer L., Dhaese P., Schreier P. H., Schmalenbach W., Van Montagu M., Schell J. Size, location and polarity of T-DNA-encoded transcripts in nopaline crown gall tumors; common transcripts in octopine and nopaline tumors. Cell. 1983 Apr;32(4):1045–1056. doi: 10.1016/0092-8674(83)90289-1. [DOI] [PubMed] [Google Scholar]
- Wing D., Koncz C., Schell J. Conserved function in Nicotiana tabacum of a single Drosophila hsp70 promoter heat shock element when fused to a minimal T-DNA promoter. Mol Gen Genet. 1989 Oct;219(1-2):9–16. doi: 10.1007/BF00261151. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zambryski P. Basic processes underlying Agrobacterium-mediated DNA transfer to plant cells. Annu Rev Genet. 1988;22:1–30. doi: 10.1146/annurev.ge.22.120188.000245. [DOI] [PubMed] [Google Scholar]
- Zambryski P., Tempe J., Schell J. Transfer and function of T-DNA genes from agrobacterium Ti and Ri plasmids in plants. Cell. 1989 Jan 27;56(2):193–201. doi: 10.1016/0092-8674(89)90892-1. [DOI] [PubMed] [Google Scholar]